Key Points
-
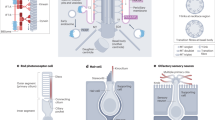
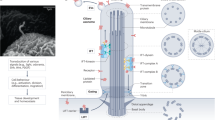
Cilia are microtubule-based hair-like organelles that extend from the surface of almost all cell types of the human body. They have been adapted as versatile tools for various tissue-specific (motile and sensory) functions during development, morphogenesis and homeostasis, explaining why cilia-related disorders (ciliopathies) can affect many organ systems.
-
During early embryonic development, the rotational movement of nodal cilia at the ventral pole of the murine embryo creates a leftward fluid flow (nodal flow), which is involved in determination of the left–right body asymmetry. However, current nodal flow hypotheses cannot completely explain the complex laterality defects (heterotaxia) that are observed in humans and mice with inborn ciliary motility defects.
-
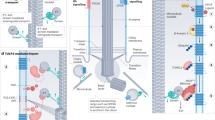
Sensory cilia act as cellular antennae to sense environmental and morphogenic cues, for example, during development. Current concepts of cilia-mediated signalling mechanisms comprise the Hedgehog signalling pathway, the Wnt/planar cell polarity pathways, receptor-mediated signalling and mechanosensory mechanisms.
-
Ciliary dysfunction is the cause of an increasing number of single organ diseases and complex syndromic forms including hydrocephalus, infertility, airway diseases, polycystic diseases of the kidney, liver and pancreas, as well as retinal diseases and defects of hearing and smell.
-
In contrast to other cell organelles, cilia are usually only assembled when cells reach a stationary or quiescent and/or differentiated state, and re-entry into the cell cycle is preceded by ciliary resorption. Therefore, cilia assembly–disassembly seems to be closely linked to cell-cycle regulation, and malfunction of these processes is involved in oncogenesis.
-
The identification of the components involved in cilia-specific functions and of the molecular mechanisms underlying the various ciliopathies are likely to facilitate the development of novel therapeutic strategies.
Abstract
Defects in the function of cellular organelles such as peroxisomes, lysosomes and mitochondria are well-known causes of human diseases. Recently, another organelle has also been added to this list. Cilia — tiny hair-like organelles attached to the cell surface — are located on almost all polarized cell types of the human body and have been adapted as versatile tools for various cellular functions, explaining why cilia-related disorders can affect many organ systems. Several molecular mechanisms involved in cilia-related disorders have been identified that affect the structure and function of distinct cilia types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Quarmby, L. M. & Parker, J. D. Cilia and the cell cycle? J. Cell Biol. 169, 707–710 (2005).
Fowkes, M. E. & Mitchell, D. R. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol. Biol. Cell 9, 2337–2347 (1998).
Rosenbaum, J. L. & Witman, G. B. Intraflagellar transport. Nature Rev. Mol. Cell Biol. 3, 813–825 (2002). This excellent article provides a detailed and comprehensive overview of IFT and various associated physiological aspects.
Avidor-Reiss, T. et al. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117, 527–539 (2004). These authors used phylogenetic screening by comparative genomics to identify genes that are essential for cilia formation and function. They also discuss the difference between IFT-dependent compartmentalized ciliogenesis and cytosolic ciliogenesis, which is independent from IFT.
Pazour, G. J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000). This article provides evidence that IFT is essential for primary cilia assembly and function in mammals and that defects in cilia assembly can lead to polycystic kidney disease.
Murcia, N. S. et al. The Oak Ridge polycystic kidney (orpk) disease gene is required for left–right axis determination. Development 127, 2347–2355 (2000).
Taulman, P. D., Haycraft, C. J., Balkovetz, D. F. & Yoder, B. K. Polaris, a protein involved in left–right axis patterning, localizes to basal bodies and cilia. Mol. Biol. Cell 12, 589–599 (2001).
Yoder, B. K. et al. Polaris, a protein disrupted in Orpk mutant mice, is required for assembly of renal cilium. Am. J. Physiol. Renal Physiol. 282, 541–552 (2002).
Feistel, K. & Blum, M. Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev. Dyn. 235, 3348–3358 (2006).
Dabdoub, A. & Kelley, M. W. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J. Neurobiol. 64, 446–457 (2005).
El Zein, L., Omran, H. & Bouvagnet, P. Lateralization defects and ciliary dyskinesia: lessons from algae. Trends Genet. 19, 162–167 (2003).
Ibanez-Tallon, I., Heintz, N. & Omran, H. To beat or not to beat: roles of cilia in development and disease. Hum. Mol. Genet. 12, R27–R35 (2003).
Satir, P. & Christensen, S. T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377–400 (2007).
Badano, J. L., Mitsuma, N., Beales, P. L. & Katsanis, N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125–148 (2006).
Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 69, 401–422 (2007).
Nonaka, S. et al. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998). In this elegant work the authors demonstrate that nodal cilia generate a left-directed flow of extra-embryonic fluid (nodal flow) that is involved in left–right body axis determination.
Ibanez-Tallon, I. et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13, 2133–2141 (2004). This article shows that the motility defect of ependymal cilia in Dnahc5 (also known as Mdnah5 )-mutant mice inhibits movement of cerebrospinal fluid through the aqueduct (ependymal flow), which results in hydrocephalus formation during late brain development.
Praetorius, H. A. & Spring K. R. A physiological view of the primary cilium. Annu. Rev. Physiol. 67, 515–529 (2005).
Qin, H. et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15, 1695–1699 (2005).
Huangfu, D. et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003). This work demonstrates that Hh signalling in vertebrates is dependent on IFT.
Schneider, L. et al. PDGFRaa signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861–1866 (2005). Using fibroblast cell cultures, these authors demonstrate that cilia can mediate PDGFR-based signalling.
Ross, A. J. et al. Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nature Genet. 37, 1135–1140 (2005).
Supp, D. M., Witte, D. P., Potter, S. S. & Brueckner, M. Mutation of an axonemal dynein affects left–right asymmetry in inversus viscerum mice. Nature 389, 963–966 (1997).
Nonaka, S., Shiratori, H., Saijoh, Y. & Hamada, H. Determination of left–right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96–99 (2002).
Essner, J. J. et al. Conserved function for embryonic nodal cilia. Nature 418, 37–38 (2002).
McGrath, J., Somlo, S., Makova, S., Tian, X. & Brueckner, M. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell 114, 61–73 (2003). The two-cilia-type hypothesis of left–right determination is based on this report, which predicts that sensory cilia at the periphery of the node sense fluid flow that is created by motile monocilia located in the centre of the node.
Tanaka, Y., Okada, Y. & Hirokawa, N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature 435, 172–177 (2005). This report introduces the nodal vesicular parcel model for left–right axis determination and demonstrates that cilia signalling at the node might be independent from mechanosensation.
Nakamura, T. et al. Generation of robust left–right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev. Cell. 11, 495–504 (2006).
Hornef, N. et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am. J. Respir. Crit. Care Med. 174, 120–126 (2006).
Zariwala, M. A. et al. Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation. Am. J. Respir. Crit. Care Med. 174, 858–866 (2006).
Ibanez-Tallon, I., Gorokhova, S. & Heintz, N. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum. Mol. Genet. 11, 715–721 (2002).
Kennedy, M. P. et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 115, 2814–2821 (2007).
Huangfu, D. & Anderson, K. V. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133, 3–14 (2006).
McMahon, A. P., Ingham, P. W. & Tabin, C. J. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol. 53, 1–114 (2003).
Zhang, Q. et al. Loss of the Tg737 protein results in skeletal patterning defects. Dev. Dyn. 227, 78–90 (2003).
Liu, A., Wang, B. & Niswander, L. A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103–3111 (2005).
Haycraft, C. J. et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 1, 480–488 (2005). The authors provide insights into the mechanism by which IFT connects to Hh signalling: GLI transcription factors are transported to the ciliary tip where they are converted into transcriptional activators and back to the cell body.
Huangfu, D. & Anderson, K. V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl Acad. Sci. USA 102, 11325–11330 (2005).
May, S. R. et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287, 378–389 (2005).
Litingtung, Y., Dahn, R. D., Li, Y., Fallon, J. F. & Chiang, C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418, 979–983 (2002).
te Welscher, P. et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science 298, 827–830 (2002).
Torban, E., Kor, C. & Gros, P. Van Gogh-like2 (Strabismus) and its role in planar cell polarity and convergent extension in vertebrates. Trends Genet. 20, 570–577 (2004).
Klein, T. J. & Mlodzik, M. Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 21, 155–176 (2005).
Smith, U. M. et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel–Gruber syndrome and the wpk rat. Nature Genet. 38, 191–196 (2006).
Kyttala, M. et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nature Genet. 38, 155–157 (2006).
Park, T. J., Haigo, S. L. & Wallingford, J. B. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nature Genet. 38, 303–311 (2006).
Boulter, C. et al. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc. Natl Acad. Sci. USA 98, 12174–12179 (2001).
Lu, W. et al. Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum. Mol. Genet. 10, 2385–2396 (2001).
McGlashan, S. R., Jensen, C. G. & Poole, C. A. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 54, 1005–1014 (2006).
Sapiro, R. et al. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol. Cell. Biol. 22, 6298–6305 (2002).
Chen, J., Knowles, H. J., Hebert, J. L., Hackett, B. P. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left–right asymmetry. J. Clin. Invest. 102, 1077–1082 (1998).
Torikata, C., Kijimoto, C. & Koto, M. Ultrastructure of respiratory cilia of WIC-Hyd male rats. An animal model for human immotile cilia syndrome. Am. J. Pathol. 138, 341–347 (1991).
Davy, B. E. & Robinson, M. L. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum. Mol. Genet. 12, 1163–1170 (2003).
Lechtreck, K. F. & Witman, G. B. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J. Cell Biol. 176, 473–482 (2007).
Banizs, B. et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132, 5329–5339 (2005).
Han, Y. G., Kwok, B. H. & Kernan, M. J. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 13, 1679–1686 (2003).
Verhage, H. G., Bareither, M. L., Jaffe, R. C. & Akbar, M. Cyclic changes in ciliation, secretion and cell height of the oviductal epithelium in women. Am. J. Anat. 156, 505–521 (1979).
Donnez, J., Casanas-Roux, F., Caprasse, J., Ferin, J. & Thomas, K. Cyclic changes in ciliation, cell height, and mitotic activity in human tubal epithelium during reproductive life. Fertil. Steril. 43, 554–559 (1985).
Teilmann, S. C. & Christensen, S. T. Localization of the angiopoietin receptors Tie-1 and Tie-2 on the primary cilia in the female reproductive organs. Cell Biol. Int. 29, 340–346 (2005).
Teilmann, S. C. et al. Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol. Reprod. Dev. 71, 444–452 (2005).
Teilmann, S. C., Clement, C. A., Thorup, J., Byskov, A. G. & Christensen, S. T. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J. Endocrinol. 191, 525–535 (2006).
Lyons, R. A., Saridogan, E. & Djahanbakhch, O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update 12, 363–372 (2006).
Afzelius, B. A. Cilia-related diseases. J. Pathol. 204, 470–477 (2004).
Van's Gravesande, K. S. & Omran, H. Primary ciliary dyskinesia: clinical presentation, diagnosis and genetics. Ann. Med. 37, 439–449 (2005).
Chilvers, M. A., Rutman, A. & O'Callaghan, C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunol. 112, 518–524 (2003).
Zariwala, M. A., Knowles, M. R. & Omran, H. Genetic defects in ciliary structure and function. Annu. Rev. Physiol. 69, 423–450 (2007).
Fliegauf, M. et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 171, 1343–1349 (2005).
Hou, Y. et al. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 176, 653–665 (2007).
Qin, H., Diener, D. R., Geimer, S., Cole, D. G. & Rosenbaum, J. L. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164, 255–266 (2004).
Budny, B. et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum. Genet. 120, 171–178 (2006).
Moore, A. et al. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J. Med. Genet. 43, 326–333 (2006).
Cano, D. A., Murcia, N. S., Pazour, G. J. & Hebrok, M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131, 3457–3467 (2004).
Zhang, Q., Davenport, J. R., Croyle, M. J., Haycraft, C. J. & Yoder, B. K. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Lab. Invest. 85, 45–64 (2005).
Masyuk, T. V. et al. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology 125, 1303–1310 (2003).
Huang, B. Q. et al. Isolation and characterization of cholangiocyte primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol. 291, 500–509 (2006).
Hildebrandt, F. & Otto, E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nature Rev. Genet. 6, 928–940 (2005).
Praetorius, H. A. & Spring, K. R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 184, 71–79 (2001). The mechanosensory model that predicts cilia-mediated signalling by passive bending is based on these observations. They show an increase of intracellular Ca2+ in response to mechanical cilia bending.
Nauli, S. M. et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genet. 33, 129–137 (2003). The hypothesis that polycystin-1 and -2 constitute a Ca2+ channel within the membrane of renal monocilia sensing mechanical stress (urinary flow) is based on this report.
Simons, M. et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genet. 37, 537–543 (2005). This work provides evidence that cilia are involved in Wnt/PCP signalling and proposes that inversin acts as a molecular switch between canonical and non-canonical Wnt pathways.
Masyuk, A. I. et al. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131, 911–920 (2006).
Saadi-Kheddouci, S. et al. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the β-catenin gene. Oncogene 20, 5972–5981 (2001).
Schwarz-Romond, T. et al. The ankyrin repeat protein diversin recruits casein kinase Iepsilon to the β-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 16, 2073–2084 (2002).
Simons, M. & Walz, G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 70, 854–864 (2006).
Kim, J. C. et al., The Bardet–Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nature Genet. 36, 462–470 (2004).
Nachury, M. V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007). This report demonstrates that several BBS proteins assemble into a core complex (the BBSome) and act in a common pathway at the ciliary base, which is involved in ciliary protein trafficking.
Benzing, T. & Walz, G. Cilium-generated signaling: a cellular GPS? Curr. Opin. Nephrol. Hypertens. 15, 245–249 (2006).
Marszalek, J. R. et al. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell 102, 175–187 (2000). This work demonstrates that the IFT-dependent transport of components of the outer segments occurs through the connecting cilium and that loss of KIF3A ultimately causes retinitis pigmentosa owing to apoptotic photoreceptor cell death.
Pazour, G. J. et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol. 157, 103–113 (2002).
Hong, D. H. et al. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest. Ophthalmol. Vis. Sci. 44, 2413–2421 (2003).
Khanna, H. et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J. Biol. Chem. 280, 33580–33587 (2005).
Roepman, R. et al. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc. Natl Acad. Sci. USA 102, 18520–18525 (2005).
Otto, E. A. et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior–Loken syndrome and interacts with RPGR and calmodulin. Nature Genet. 37, 282–288 (2005).
Chang, B. et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 15, 1847–1857 (2006).
Katsanis, N. et al. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet–Biedl syndrome. Nature Genet. 26, 67–70 (2000).
Beales, P. L. Lifting the lid on Pandora's box: the Bardet–Biedl syndrome. Curr. Opin. Genet. Dev. 15, 315–323 (2005).
Maffei, P., Munno, V., Marshall, J. D., Scandellari, C. & Sicolo, N. The Alstrom syndrome: is it a rare or unknown disease? Ann. Ital. Med. Int. 17, 221–228 (2002).
Collin, G. B. et al. Alms1-disrupted mice recapitulate human Alstrom syndrome. Hum. Mol. Genet. 14, 2323–2333 (2005).
Bonneau, D. et al. Usher syndrome type I associated with bronchiectasis and immotile nasal cilia in two brothers. J. Med. Genet. 30, 253–254 (1993).
Reiners, J. et al. Differential distribution of harmonin isoforms and their possible role in Usher-1 protein complexes in mammalian photoreceptor cells. Invest. Ophthalmol. Vis. Sci. 44, 5006–5015 (2003).
Reiners, J., Nagel-Wolfrum, K., Jurgens, K., Marker, T. & Wolfrum, U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp. Eye Res. 83, 97–119 (2006).
Wolfrum, U. & Schmitt, A. Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil. Cytoskeleton 46, 95–107 (2000).
Kulaga, H. M. et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nature Genet. 36, 994–998 (2004).
Iannaccone, A. et al. Clinical and immunohistochemical evidence for an X linked retinitis pigmentosa syndrome with recurrent infections and hearing loss in association with an RPGR mutation. J. Med. Genet. 40, e118 (2003).
Kim, J. et al. A TRPV family ion channel required for hearing in Drosophila. Nature 424, 81–84 (2003).
Adato, A. et al. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet. 14, 3921–3932 (2005).
Qin, H., Wang, Z., Diener, D. & Rosenbaum, J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr. Biol. 17, 193–202 (2007). The authors show in C. reinhardtii that components of the IFT machinery are directly involved in cell-cycle control and demonstrate the close relationship between ciliogenesis and cell division.
Robert, A. et al. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1–S transition in non-ciliated cells. J. Cell Sci. 120, 628–637 (2007).
Pugacheva, E. N., Jablonski, S. A., Hartman, T. R., Henske, E. P. & Golemis, E. A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351–1363 (2007). This report shows that activation of Aurora A (a centrosome-associated kinase that regulates mitotic entry and mitotic spindle organization) induces ciliary resorption by a mechanism that involves phosphorylation of HDAC6 and stimulation of HDAC6-dependent tubulin deacetylation.
Brown, J. M., Marsala, C., Kosoy, R. & Gaertig, J. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol. Biol. Cell 10, 3081–3096 (1999).
Mahjoub, M. R., Qasim Rasi, M. & Quarmby, L. M. A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol. Biol. Cell. 15, 5172–5186 (2004).
Mahjoub, M. R., Trapp, M. L. & Quarmby, L. M. NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J. Am. Soc. Nephrol. 16, 3485–3489 (2005).
Quarmby, L. M. & Mahjoub, M. R. Caught Nek-ing: cilia and centrioles. J. Cell Sci. 118, 5161–5169 (2005).
Upadhya, P., Birkenmeier, E. H., Birkenmeier, C. S. & Barker, J. E. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc. Natl Acad. Sci. USA 97, 217–221 (2000).
Liu, S. et al. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development 129, 5839–5846 (2002).
Li, X. et al. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nature Cell Biol. 7, 1202–1212 (2005).
Bhunia, A. K. et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109, 157–168 (2002). This article describes a role of the polycystin-1/-2 complex in the regulation of the cell cycle by JAK/STAT pathway activation and p21 upregulation.
Chauvet, V. et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 114, 1433–1443 (2004).
Esteban, M. A., Harten, S. K., Tran, M. G. & Maxwell, P. H. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J. Am. Soc. Nephrol. 17, 1801–1806 (2006).
Lutz, M. S. & Burk, R. D. Primary cilium formation requires von Hippel-Lindau gene function in renal-derived cells. Cancer Res. 66, 6903–6907 (2006).
Schermer, B. et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J. Cell Biol. 175, 547–554 (2006). These authors show that the tumour-suppressor protein VHL is a ciliary protein involved in ciliogenesis, linking tumorigenesis to cilia dysfunction.
O'Toole, E. T., Giddings, T. H., McIntosh, J. R. & Dutcher, S. K. Three-dimensional organization of basal bodies from wild-type and δ-tubulin deletion strains of Chlamydomonas reinhardtii. Mol. Biol. Cell. 14, 2999–3012 (2003).
Dutcher, S. K. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic 4, 443–451 (2003).
Bukanov, N. O., Smith, L. A., Klinger, K. W., Ledbetter, S. R. & Ibraghimov-Beskrovnaya, O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444, 949–952 (2006).
Olbrich, H. et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nature Genet. 34, 455–459 (2003).
Omori, S. et al. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J. Am. Soc. Nephrol. 17, 1604–1614 (2006).
Torres, V. E. & Harris, P. C. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nature Clin. Pract. Nephrol. 2, 40–55 (2006).
Astrinidis, A., Senapedis, W. & Henske, E. P. Hamartin, the tuberous sclerosis complex 1 gene product, interacts with polo-like kinase 1 in a phosphorylation-dependent manner. Hum. Mol. Genet. 15, 287–297 (2006).
Tao, Y., Kim, J., Schrier, R. W. & Edelstein, C. L. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J. Am. Soc. Nephrol. 16, 46–51 (2005).
Cano, D. A., Sekine, S. & Hebrok, M. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology 131, 1856–1869 (2006).
Scholey, J. M. & Anderson, K. V. Intraflagellar transport and cilium-based signaling. Cell 125, 439–442 (2006).
Acknowledgements
We thank E. Davis and G. Pazour for critical evaluation of the manuscript. H.O. and M.F. are supported by the Deutsche Forschungs-Gemeinschaft. We are grateful for the collaboration with the patient support group 'PCD und Kartagener Syndrom e.V.'. We thank H. Olbrich and N.T. Loges for help with figure preparations.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary informations The supplementary movies demonstrate normal sperm motility in comparison with immotile and dysmotile sperm flagella. Also shown are normal respiratory cilia function and different aberrant beating patterns of respiratory cilia, which are typically observed in patients with primary ciliary dyskinesia (PCD) and are fundamental for diagnosis. Supplementary information S1 (movie)
Human sperm cells with normal motility (AVI 3141 kb)
Supplementary information S2 (movie)
Immotile sperm cells from a PCD patient (AVI 2938 kb)
Supplementary information S3 (movie)
Dysmotile sperm cells from a PCD patient (AVI 3437 kb)
Supplementary information S4 (movie)
Human respiratory epithelial cell layer with normal ciliary beating (AVI 2559 kb)
Supplementary information S5 (movie)
Human respiratory epithelial cells with normal ciliary beating (AVI 1071 kb)
Supplementary information S6 (movie)
Immotile respiratory cilia from a PCD patient with DNAH5 mutations (AVI 1405 kb)
Supplementary information S7 (movie)
Dysmotile respiratory cilia from a PCD patient with DNAI1 mutations (AVI 2230 kb)
Supplementary information S8 (movie)
Dysmotile respiratory cilia from a PCD patient with unknown mutations (AVI 1074 kb)
Supplementary information S9 (movie)
Uncoordinated beating of respiratory cilia from a PCD patient with OFD1 mutations (AVI 1153 kb)
Related links
Glossary
- Intraflagellar transport
-
(IFT). A cilia-specific and flagella-specific transport system that relies on at least 16 different proteins that assemble into transport rafts and move ciliary components across the compartment border and along the peripheral axonemal microtubules to the ciliary tip and back to the cell body. IFT was first described in bi-flagellate green algae (Chlamydomonas reinhardtii).
- Ciliogenesis
-
The processes of cilia assembly and growth that follow and/or accompany cell polarization.
- Notochordal plate
-
An epithelial primordial structure of the notochord (a cylindrical rod of cells). The sheet of notochordal cells is laterally in contact with the roof of the primitive gut and dorsally in contact with the midline cells of the neural plate. The notochordal plate folds off from the roof of the primitive gut to form the notochord.
- Anterograde
-
The transport direction from the ciliary base to the tip.
- Retrograde
-
The transport direction from the ciliary tip back to the cell body.
- Mucociliary clearance
-
The process by which the continuous coordinated beating of respiratory cilia moves the thin mucus layer that covers the airway epithelia towards the pharynx to defend against inhaled pathogens trapped in the mucus.
- Ependymal flow
-
The laminar flow of cerebrospinal fluid through the brain ventricles and the cerebral aqueduct generated by the coordinated beating of ependymal cilia.
- Primary ciliary dyskinesia
-
(PCD). A genetically and phenotypically heterogeneous group of disorders characterized by defective ciliary motility.
- Nephronophthisis
-
An autosomal recessive cystic kidney disease characterized by normal or reduced kidney size, cysts at the corticomedullary border and predominant tubulointerstitial fibrosis. Phthisis is a Greek word meaning shrinking or wasting.
- Situs inversus totalis
-
The complete mirror-image arrangement of all thoracic and abdominal organs.
- Situs solitus
-
The normal position of the viscera (stomach and spleen on the left side, liver on the right side). The three-lobed lung is positioned on the right, with the two-lobed lung on the left, and the left and right cardiac atria are positioned normally.
- Situs inversus abdominalis
-
The isolated inversion of abdominal organs, but a normal composition of thoracic organs.
- Situs inversus thoracalis
-
The isolated inversion of thoracic organs, but a normal composition of abdominal organs.
- Holoprosencephaly
-
A developmental disorder of the brain due to a failure of the embryonic forebrain (the prosencephalon) to form bilateral hemispheres of the cephalon. This causes defects in brain structure and function and also affects the development of the face.
- Polydactyly
-
Supernumerary fingers or toes. The presence of six fingers or six toes on one or both hands or feet is usually called hexadactyly.
- Craniofacial defects
-
The developmental abnormalities that affect the head or skull and structures of the face.
- Bardet–Biedl syndrome
-
(BBS). A clinically pleiotropic disorder that has a primarily autosomal recessive inheritance pattern (twelve loci, BBS1–BBS12, have been identified so far) and a multitude of symptoms including rod–cone dystrophy, retinitis pigmentosa, obesity, polydactyly, renal abnormalities (such as cystic kidneys), learning disabilities or mental retardation, male hypogonadism and congenital heart defects. BBS proteins localize either to the ciliary base or the axoneme and are involved in subcellular targeting of ciliary proteins. Seven of the known BBS proteins assemble into a core complex called the BBSome.
- Spina bifida
-
A developmental abnormality that results from an incomplete closure of the embryonic neural tube and an incompletely formed spinal cord that protrudes through an open gap in the unfused spines of the vertebrae (spina bifida aperta). In the milder form, spina bifida occulta, the spinal cord does not protrude because only a small part of one vertebra is missing and there is no opening to the skin.
- Osteochondro-dysplasia
-
An abnormal growth of cartilage and bone (individual bones or group of bones). Growth defects of the long bones and/or spine usually cause shortened limbs or a disproportionately shortened body.
- Metachronal wave
-
A wave-like movement that is propagated along the epithelial surface, created when cilia on one segment of the epithelium move after another.
- Cholangiocytes
-
The epithelial cells of the bile ducts.
- Bronchiectasis
-
A bag-like or cylindrical widening of parts of the bronchial tree, which is usually caused by localized injury of bronchial tissue due to bacterial infections. Affected bronchi are irreversibly damaged.
- Chronic sinusitis
-
A permanent or recurrent inflammation of the paranasal sinuses often caused by infections.
- Hypoosmia
-
A decreased ability to smell odours.
- Anosmia
-
The absence of the ability to smell odours.
Rights and permissions
About this article
Cite this article
Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8, 880–893 (2007). https://doi.org/10.1038/nrm2278
Issue Date:
DOI: https://doi.org/10.1038/nrm2278
This article is cited by
-
The peroxisome: an update on mysteries 3.0
Histochemistry and Cell Biology (2024)
-
Joubert syndrome causing mutation in C2 domain of CC2D2A affects structural integrity of cilia and cellular signaling molecules
Experimental Brain Research (2024)
-
IK is essentially involved in ciliogenesis as an upstream regulator of oral-facial-digital syndrome ciliopathy gene, ofd1
Cell & Bioscience (2023)
-
Mucociliary Wnt signaling promotes cilia biogenesis and beating
Nature Communications (2023)
-
Integrated analysis of copy number variation-associated lncRNAs identifies candidates contributing to the etiologies of congenital kidney anomalies
Communications Biology (2023)