-
PDF
- Split View
-
Views
-
Cite
Cite
Merav Cohen-Lahav, Shraga Shany, David Tobvin, Cidio Chaimovitz, Amos Douvdevani, Vitamin D decreases NFκB activity by increasing IκBα levels, Nephrology Dialysis Transplantation, Volume 21, Issue 4, April 2006, Pages 889–897, https://doi.org/10.1093/ndt/gfi254
Close - Share Icon Share
Abstract
Background. In a previous study we demonstrated the inhibitory effect of 1,25-dihydroxyvitamin D (1,25(OH)2D3) and its less calcaemic analog 1,24(OH)2D2 on the production of tumour necrosis factor alpha (TNFα) by human peritoneal macrophages. The aim of the present study is to examine whether this vitamin D inhibition of TNFα is mediated by its major transcription factor, nuclear factor-κB (NFκB).
Methods. Murine macrophage cells (P388D1) were incubated with 10−7 M 1,25(OH)2D3 or 1,24(OH)2D2 and then stimulated with lipopolysaccharide. NFκB activity was assayed using a reporter gene and by electrophoretic mobility shift assay (EMSA). In addition, we evaluated mRNA and protein levels of NFκB-p65 and of IκBα, a potent NFκB inhibitor, and phosphorylated IκBα.
Results. Both 1,25(OH)2D3 and 1,24(OH)2D2 induced a 60% reduction of TNFα secretion. By using a reporter gene and EMSA we found that vitamin D markedly reduced NFκB activity. 1,25(OH)2D3 or 1,24(OH)2D2 decreased NFκB-p65 levels in the nucleus and increased NFκB-p65 levels in the cytosol; no changes were observed in the total levels of NFκB-p65 protein and mRNA. Concurrently, vitamin D induced a significant increase in mRNA and protein levels of IκBα (∼6.5- and 4.5-fold, respectively). Elevated levels of IκBα can be explained by the vitamin D-induced prolongation of IκBα-mRNA half-life from 110 to 190 min and by the decrease in IκBα phosphorylation.
Conclusions. Vitamin D up-regulates IκBα levels by increasing mRNA stability and decreasing IκBα phosphorylation. The increase in IκBα levels reduces nuclear translocation of NFκB and thereby downgrades its activity. Since NFκB is a major transcription factor of inflammatory mediators, these findings suggest that the less-calcaemic analog, 1,24(OH)2D2 may be effective as an anti-inflammatory therapeutic agent.
Introduction
The active metabolite of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D3) can be produced not only by the kidney, but also by macrophages, including peritoneal macrophages, especially during inflammation [1]. Furthermore, it has been well established that the physiological role of 1,25(OH)2D3 is not limited to mineral and skeletal homeostasis, but that it also plays a role as an important immune response regulator at inflammatory sites [2]. In a previous study we demonstrated a dose-dependent inhibitory effect of 1,25(OH)2D3 and its less calcaemic analog 1,24(OH)2D2 on both tumour necrosis factor alpha (TNFα) mRNA and protein levels in lipopolysaccharide (LPS)-stimulated human peritoneal macrophages obtained from effluent dialysates of patients in end-stage renal disease undergoing continuous ambulatory peritoneal dialysis and by the murine macrophage cell line P388D1 [3,4]. TNFα is a major mediator of host response to pathogens, in that it initiates a powerful proinflammatory cascade which promotes massive recruitment of leukocytes at the infected site. However, excessive production of TNFα and protracted or over-activated inflammation are the hallmarks of systemic inflammatory response syndrome as well as inflammatory diseases such as Crohn's disease and rheumatoid arthritis. Therefore, regulation of TNFα production may have significant clinical importance [5,6].
The promoter region of the TNFα gene contains a complex array of potential regulatory elements. However, the role of these regulatory elements in transcriptional activation of the TNFα gene in human monocytes and macrophages remains unclear [7]. Transcriptional activation of the TNFα gene in macrophages has been demonstrated to be predominantly dependent on the transcription factor nuclear factor-κB (NFκB), which has four binding sites in the TNFα promoter [6,8].
NFκB is a major regulator of gene transcription involved in immune, inflammatory and stress responses. It consists of five proteins which tend to dimerize and are thus kept in the cytoplasm through interaction with IκBα inhibitory proteins. The most dominant protein in the NFκB family is the p65 protein and the best-characterized interaction is that of the transcriptionally active p65 with p50 which does not possess a transactivation domain [9]. NFκB is normally sequestered in the cytoplasm of non-stimulated cells and consequently must be translocated into the nucleus to function. The subcellular location of NFκB is controlled by a family of inhibitory proteins called IκBs [10]. One of the most important regulators of mammalian NFκB is IκBα. When IκBα binds to the p50/p65 heterodimer complex, it maintains the heterodimers in the cytoplasm and prevents activation of NFκB target gene transcription [10]. In response to cell stimulation, such as LPS, IκBα undergoes rapid phosphorylation leading to polyubiquitination and 26S proteasome-mediated degradation, allowing NFκB to translocate into the nucleus and to activate target genes [10]. Our aim in this study is to examine if the inhibitory effect of 1,25(OH)2D3 and 1,24(OH)2D2 on TNFα production in the macrophage cell line P388D1 is mediated through reduction of NFκB activity and to find a possible mechanism for the inhibitory effect. Since current anti-inflammatory therapeutic agents, such as steroids or anti-TNF antibodies, have significant side effects, therapeutic use of the less hypercalcaemic analog, 1,24(OH)2D2, as an anti-inflammatory agent may provide a breakthrough in pharmaceutical application [2].
Subjects and methods
Materials
1,25(OH)2D3 was kindly provided by Hoffman La-Roche, (Basel, Switzerland) and 1,24(OH)2D2 by Bone Care International Co. (Madison, WI, USA).
Methods
Incubation of cells
P388D1 cells, a murine macrophage cell line, were treated with 1,25(OH)2D3, (10−7 M) or 1,24(OH)2D2 (10−7 M) for 16 h in RPMI medium containing 2% FCS. Then they were exposed to 1 µg/ml of LPS (E. coli 055:B5 lipopolysaccharide LPS, Sigma), for 8 h for luciferase assay, 6 h for TNFα protein determination by ELISA, 2.5 h for NFκB and IκBα-mRNA determination by reverse transcriptase-polymerase chain reaction (RT-PCR) or for 0.5 h for NFκB and IκBα protein measurement by western blot and electrophoretic mobility shift assay (EMSA).
TNFα protein determination
TNFα protein levels were determined by ELISA (R&D Systems, Minneapolis, MN, USA).
Plasmid DNA purification system
We used the Wizard PureFection Plasmid DNA Purification System (Promega, Madison, WI, USA), which includes a proprietary endotoxin removal resin.
Transfection and luciferase assay
P388D1 cells were transfected by electroporation (350 V, 1500 μF, Easyject optima). With 1.5 µg of reporter plasmid pNFκB-Luc (Clontech, Palo Alto, CA), 5 × 106 cells were transfected. After transfection, cells were seeded into six-well plates (250 × 103/well). After 24 h of incubation, cells were treated with 1,25(OH)2D3 (10−7 M) or 1,24(OH)2D2 (10−7 M) for 16 h and activated with LPS (1 µg/ml) for an additional 8 h. Finally, the medium of each sample was collected and assayed for TNFα. Simultaneously, the cells were harvested for luciferase assay. Cells were lysed by lysis buffer (Promega) and one freeze–thaw cycle. Supernatants containing cell extracts were obtained after centrifugation for 5 min at 13 000 g and stored at −70°C. The assay to determine NFκB activity was performed for each sample by injecting 100 μl of substrate solution to 20 μl of cell extract by a luminometer. Luciferase activity was normalized by total protein concentration and variations in transfection efficiency.
Cell lysate preparation
P388D1 cells were treated with 1,25(OH)2D3 or 1,24(OH)2D2 as described previously, followed by stimulation with 1 µg/ml of LPS for an additional 0.5 h. After the stimulation period, whole-cell lysate, cytoplasmatic and nuclear extracts were obtained according to the procedure described by Jiang et al. [11]. The purity of the fractions was confirmed by western blot using an anti-actin (Oncogen, Boston, MA) which is specific for the cytosolic fraction and by an anti-PCNA (BD Biosciences, USA) a specific marker for the nuclear fraction. The lysis buffer we used contained in addition to a protease inhibitor mixture (Sigma-Aldrich, Rehovot, Israel) two phosphatase inhibitors (Na3VO4 and NaF).
EMSA
Nuclear extracts from P388D1 cells were prepared as described previously. The synthetic double-stranded oligonucleotide containing the NFκB binding sequence (in the HIV-LTR) was as follows: 5′-AAT TTC CCT GGA AAG TCC CCA GCG GAA AGT CCC TTG T-3′ [12]. The oligonucleotide and its complementary strand were annealed and end-labelled by T4 kinase-mediated phosphorylation reaction in the presence of [γ-32P] ATP. 100 000 cpm of a [γ-32P] end-labelled probe was incubated with 20 µg of nuclear protein extract for 30 min at room temperature in a total volume of 30 μl of reaction buffer (1 µg/μl of poly (dI-dC) (Sigma-Aldrich) 25 mM Hepes, pH 7.9, 100 mM NaCl, 333 mM urea, 10% glycerol, 0.33% NP-40, 1 mM DTT and 5 µg acetylated BSA). Competition studies were performed by incubating nuclear extracts with an excess (100-fold) unlabelled NFκB oligonucleotide for 15 min at room temperature, followed by 30 min incubation in the presence of the probe. In addition, we inhibited the binding of the NFκB-p65 protein by incubating the nuclear protein lysate for 60 min on ice with 1 μl of polyclonal competing antibody directed against p65 (anti-NFκB p65, CT Upstate Biotechnology, Charlottesville, VA, USA). Samples were subjected to electrophoresis on a 7% non-denaturating polyacrilamide gel at 4°C for 1.5 h. The gels were dried and exposed to X-ray film. To quantitate the amount of NFκB proteins specifically bound to the probe, the intensity of the bands was calculated using densitometric analysis.
Western blot
P388D1 cells were treated with 1,25(OH)2D3 or 1,24(OH)2D2 (10−7 M) for 16 h followed by an additional 0.5 h of incubation with LPS (1 µg/ml). At the end of the incubation period the cells were lysed and nucleus and cytosol fractions were prepared for the western blot procedure. The extracts were subjected to electrophoresis in SDS-PAGE 12.5% and transferred to a nitrocellulose membrane (Gellman, Italy). The primary antibodies used were the rabbit polyclonal antibodies of anti-IκBα (Santa Cruz, , CA, USA), and anti-NFκB-p65 (UBI-Upstate Biotechnology, NY, USA), at a 1/1000 dilution. To detect the phosphorylation of Ser32 of IκBα, anti-phosphoserine-IκBα monoclonal antibody was used (mouse monoclonal antibodies anti-phospho-Ser32-IκBα Santa Cruz) at a 1/1000 dilution.
NFκB and IκBα-mRNA analysis
NFκB-P65-mRNA and IκBα-mRNA were determined by RT-PCR of total RNA extracted from P388D1 cells. Cells were incubated as described above and total RNA was extracted from the cells with the RNeasy Mini Kit (Qiagen). For RNA decay analysis, cells were treated with or without 1,25(OH)2D3 and 1,24(OH)2D2 (10−7 M). Subsequently, actinomycin-D (Sigma) was added (1 µg/ml) and the cells were incubated for various lengths of time followed by RNA extraction.
NFκB and IκBα RT-PCR and real-time PCR
For cDNA generation, 13 μl of RNA sample was added to each 7 μl of reverse transcriptase reaction mixture, as described in [3]. cDNA was then amplified by PCR using specific primers (Table 1). To 45 μl of PCR reaction mixture (Ready Mix PCR Master Mix, ABgene, and Surrey, UK), 5 μl of reverse transcription product was added as per the protocol of the manufacturer. Thirty cycles for NFκB-p65, 25 cycles for IκBα and 25 cycles for β-actin were in the exponential phase of amplification in most of the experiments, thus permitting comparison of mRNA levels in different samples. On an agarose gel, 8 μl of each sample containing amplified cDNA were loaded as previously described [3]. NFκB-p65 and IκBα levels were normalized according to β-actin levels in order to compensate for differences in mRNA between the various samples.
List of primers used for RT-PCR DNA amplification
| Gene . | Primer sequence . | Product size (bp) . |
|---|---|---|
| NFκB-P65 | 5′-ATGGACGATCTGTTTCCCCT-3′ | 602 |
| 5′-CGGTTTACTCGGCAGATCTT-3′ | ||
| IκBα | 5′-GATGGCCTCAAGAAGGAGCGCT-3′ | 469 |
| 5′-AGTGGAGATGCTGGGGTGTGCA-3′ | ||
| β-actin | 5′-GGTCTCAAACATGATCTGGG-3′ | 237 |
| 5′-GGGTCAGAAGGATTCCTATG-3′ |
| Gene . | Primer sequence . | Product size (bp) . |
|---|---|---|
| NFκB-P65 | 5′-ATGGACGATCTGTTTCCCCT-3′ | 602 |
| 5′-CGGTTTACTCGGCAGATCTT-3′ | ||
| IκBα | 5′-GATGGCCTCAAGAAGGAGCGCT-3′ | 469 |
| 5′-AGTGGAGATGCTGGGGTGTGCA-3′ | ||
| β-actin | 5′-GGTCTCAAACATGATCTGGG-3′ | 237 |
| 5′-GGGTCAGAAGGATTCCTATG-3′ |
List of primers used for RT-PCR DNA amplification
| Gene . | Primer sequence . | Product size (bp) . |
|---|---|---|
| NFκB-P65 | 5′-ATGGACGATCTGTTTCCCCT-3′ | 602 |
| 5′-CGGTTTACTCGGCAGATCTT-3′ | ||
| IκBα | 5′-GATGGCCTCAAGAAGGAGCGCT-3′ | 469 |
| 5′-AGTGGAGATGCTGGGGTGTGCA-3′ | ||
| β-actin | 5′-GGTCTCAAACATGATCTGGG-3′ | 237 |
| 5′-GGGTCAGAAGGATTCCTATG-3′ |
| Gene . | Primer sequence . | Product size (bp) . |
|---|---|---|
| NFκB-P65 | 5′-ATGGACGATCTGTTTCCCCT-3′ | 602 |
| 5′-CGGTTTACTCGGCAGATCTT-3′ | ||
| IκBα | 5′-GATGGCCTCAAGAAGGAGCGCT-3′ | 469 |
| 5′-AGTGGAGATGCTGGGGTGTGCA-3′ | ||
| β-actin | 5′-GGTCTCAAACATGATCTGGG-3′ | 237 |
| 5′-GGGTCAGAAGGATTCCTATG-3′ |
Quantitative real-time PCR assays were carried out for NFκB-p65, IκBα and β-actin. Templates (7 µl) were diluted 5-fold and then mixed with primers (0.2 mM) and Thermo-Start master mix (ABgene). SYBR green I dye (Amresco, Inc. USA) was added to the reaction mixture. Reactions were carried out in the Rotor-Gene Real-Time Analysis Software PCR machine (Corbett-Research). Standard cycling conditions for this instrument were used: 15 min initial denaturation at 95°C, then 40 cycles denaturation at 95°C for 15 or 20 s; annealing (the exact temperature was determined according to the primers) for 15 or 20 sec; extension at 72°C for 20 s. A melting curve was then sketched to ensure the purity of the amplified product.
The efficiency of the real-time PCR reactions was between 0.95 and 1.01.
Statistical analysis
Results are presented as mean±standard error of the mean (SE). ANOVA was used to compare TNFα, NFκB and IκBα levels between treatments. P-values below 0.05 were considered significant.
Results
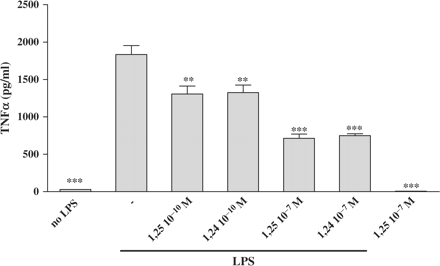
1,25(OH)2D3 and 1,24(OH)2D2 decrease TNFα protein levels.
TNFα protein levels were examined in P388D1 cells media using a specific ELISA. Pre-incubation of P388D1 cells for 16 h with increasing concentrations (10−10–10−7 M) of 1,25(OH)2D3 or 1,24(OH)2D2 (not all concentrations are shown), followed by stimulation with LPS (1 µg/ml) for an additional 6 h, induced a statistically significant dose-related decrement in TNFα protein concentrations (Figure 1). TNFα secretion was downregulated significantly (29% inhibition, P<0.01) in the presence of a physiological concentration (10−10 M) of 1,25(OH)2D3 and reached a maximal effect (61% inhibition, P<0.001) with higher concentration of 10−8 M (data not shown) or 10−7 M of 1,25(OH)2D3/1,24(OH)2D2.
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 reduces TNFα protein secretion. P388D1 (1 × 106 cells/well) were incubated for 16 h with increased concentrations of 1,25(OH)2D3 or 1,24(OH)2D2 (10−10 to 10−7 M). This was followed by an additional 6 h of incubation with LPS (1 µg/ml). Supernatants were collected and subsequently assayed for TNFα protein levels by ELISA. The results are expressed as mean SE and represent three experiments performed in triplicate (***P<0.001, **P<0.01 vs LPS alone).
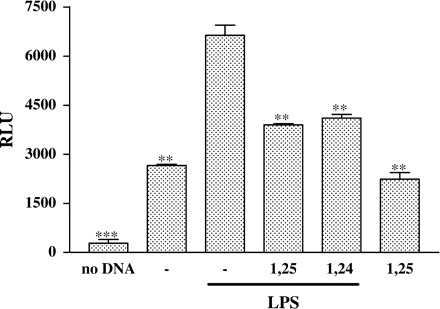
1,25(OH)2D3 and 1,24(OH)2D2 inhibit NFκB activity.
To examine the effect of 1,25(OH)2D3 and 1,24(OH)2D2 on transcriptional activity of NFκB, we transiently transfected P388D1 cells with the pNFκB-Luc construct. pNFκB-Luc contains a firefly luciferase gene that is especially designed for monitoring the activation of the NFκB signal transduction pathway. Analysis of luciferase activity in cell extracts from transfected cells confirmed that stimulation of the cells with LPS results in an increase in NFκB-driven luciferase expression (Figure 2). In the presence of 1,25(OH)2D3 or 1,24(OH)2D2, however, the increase in luciferase activity was partially inhibited (Figure 2). Similar results were obtained in separate experiments. We also found a positive correlation between inhibition of NFκB activity and decrement in TNFα protein levels induced by 1,25(OH)2D3 and 1,24(OH)2D2, as described previously (Figure 1).
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 inhibits NFκB activity. P388D1 cells were transiently transfected with the NFκB plasmid containing the luciferase reporter gene (pNFκB-Luc). 24 h later the cells were treated for 16 h with 1,25(OH)2D3 or 1,24(OH)2D2 and subsequently stimulated with LPS for an additional 8 h. Cell lysates were prepared, and luciferase activity present in 50 µg of cellular protein was measured. The results are expressed as mean±SE and represent three experiments performed in triplicate (***P<0.001, **P<0.01 vs LPS alone).
The inhibitory effect of 1,25(OH)2D3 and 1,24(OH)2D2 on NFκB activity, as shown by luciferase analysis (Figure 2), was in accordance with the results obtained from EMSA (Figure 3). Nuclear extracts of P388D1 cells were incubated with end-labelled double stranded oligonucleotide possessing the NFκB consensus site. The nuclear extracts from the LPS-stimulated P388D1 cells yielded a large band in comparison to the unstimulated cells, and exhibited DNA binding activity to the NFκB site containing oligonucleotide (Figure 3). However, the nuclear extracts of P388D1 cells treated with 1,25(OH)2D3 or 1,24(OH)2D2 caused a marked reduction in DNA-protein complex binding activity, as evidenced by a decrease in the intensity of this band.
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 decreases NFκB DNA binding activity. Cells were incubated for 16 h with 1,25(OH)2D3 or 1,24(OH)2D2 (10−7 M) and then activated with LPS (1 µg/ml) for an additional 0.5 h. Nuclear extracts (20 µg protein/lane) were incubated with [32P]-end-labelled dsDNA oligonucleotide possessing the NFκB consensus sequence, and then the ability of bound proteins to induce retarded mobility of the radiolabelled probe on polyacrilamide gel was tested. Binding specificity was tested using 100-fold excess of ‘cold’ DNA probe and nuclear proteins from the nuclear extracts of activated cells. In addition, nuclear proteins from the nuclear extracts of activated cells were incubated with a competitive inhibitory antibody directed against p65 protein or with an isotype control antibody. One representative experiment out of five is shown.
A competing antibody directed against the NFκB subunit p65 blocked the binding of the nuclear extracts to the labelled oligonucleotide indicating the specificity of this assay. However, when cells were treated with an isotype-matched control antibody, the DNA-protein complex was not affected. The labelled oligonucleotide could be competed out by 100-fold excess of specific unlabelled ‘cold’ DNA probe.
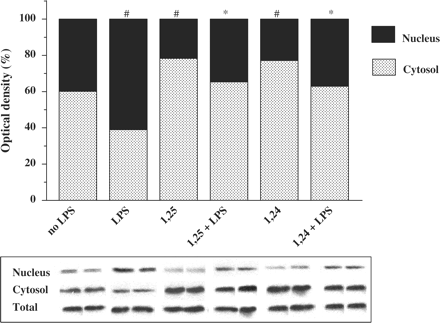
Decrease in NFκB-p65 protein levels in the nuclear compartment by 1,25(OH)2D3 and 1,24(OH)2D2 is associated with induced expression in the cytosol
After demonstrating that 1,25(OH)2D3 and 1,24(OH)2D2 inhibit NFκB activity as measured by luciferase assay (Figure 2) and EMSA (Figure 3), we tried to elucidate the mechanism responsible for this down-regulation. We found that stimulation of the cells with LPS induced an increase in NFκB-p65 protein expression in the nucleus, while in the cytosol it decreased NFκB-p65 protein expression (Figure 4). On the other hand, treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 (with or without stimulation with LPS) decreased NFκB-p65 levels in the nucleus (Figure 4) and increased NFκB-p65 levels in the cytosol (Figure 4). NFκB-p65 levels in total lysate were not affected by 1,25(OH)2D3 and 1,24(OH)2D2 (Figure 4). Decreased NFκB-p65 translocation to the nuclear compartment, resulting from treatment with 1,25(OH)2D3 or 1,24(OH)2D2 was associated with increased levels of NFκB-p65 in the cytosol (Figure 4). Treatment of cells with 1,25(OH)2D3 or 1,24(OH)2D2 without LPS caused a similar effect in NFκB-p65 expression or localization, in comparison to LPS-unstimulated cells (Figure 4).
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 reduces NFκB-p65 in the nucleus and increases NFκB-p65 in the cytosol. P388D1 cells were preincubated for 16 h with 1,25(OH)2D3 or 1,24(OH)2D2 (10−7 M) followed by activation with LPS (1 µg/ml) for an additional 0.5 h. Nuclear and cytosol extracts were prepared and western blot analysis was performed, using an anti-NFκB-p65 antibody. The results are expressed as mean±SE and represent three experiments performed in duplicate #P<0.05 vs no LPS, *P<0.05 vs LPS alone.
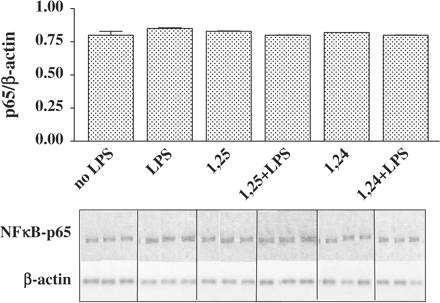
1,25(OH)2D3 and 1,24(OH)2D2 do not affect NFκB-p65 mRNA levels.
To support the finding that NFκB-p65 protein levels did not change in the total lysate, when treated by 1,25(OH)2D3 and 1,24(OH)2D2 (Figure 4), we measured NFκB-p65 mRNA levels by using specific primers (Table 1). RT-real-time PCR and RT-PCR of total RNA from P388D1 cells yielded a band of mRNA for NFκB-p65 at the expected size of 602 bp (Figure 5). 1,25(OH)2D3 and 1,24(OH)2D2 had no effect on NFκB-p65 mRNA levels.
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 does not affect p65-mRNA levels. P388D1 cells were incubated for 16 h with 1,25(OH)2D3 or 1,24(OH)2D2 (10−7 M) and then activated with LPS (1 µg/ml) for an additional 2.5 h. Subsequently, cells were lysed and RNA was extracted. RT-PCR was performed on total cell RNA, using specific primers for NFκB-p65. PCR products (at 30 cycles) were separated on 2% agarose gel containing ethidium bromide (lower panel). Results of real-time PCR analysis of NFκB-p65 mRNA levels were normalized to the levels of β-actin. The results are expressed as mean±SE and represent three experiments performed in triplicate.
1,25(OH)2D3 and 1,24(OH)2D2 increase IκBα protein levels.
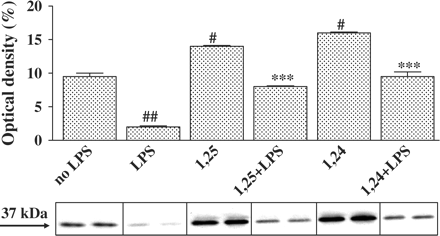
Since we found no effect on total NFκB-p65 protein (Figure 4) and NFκB-p65 mRNA levels (Figure 5) in P388D1 cells treated with 1,25(OH)2D3 or 1,24(OH)2D2 we decided to investigate if the decrease in NFκB activity is associated with its inhibitor IκBα. We found that stimulation of P388D1 cells with LPS decreased IκBα protein levels in the cytosol, whereas 1,25(OH)2D3 and 1,24(OH)2D2 significantly increased IκBα protein levels (×4.5) (Figure 6).
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 increases cytosolic IκBα levels. Cells were incubated for 16 h with 1,25(OH)2D3 or 1,24(OH)2D2 (10−7 M) and then stimulated with LPS (1 µg/ml) for an additional 0.5 h. Cytosol extract was prepared and IκBα levels were analysed by western blot analysis, using an anti-IκBα antibody. The results are expressed as mean±SE and represent three experiments performed in duplicate #P<0.05 or ##P<0.01 vs no LPS, ***P<0.001 vs LPS alone.
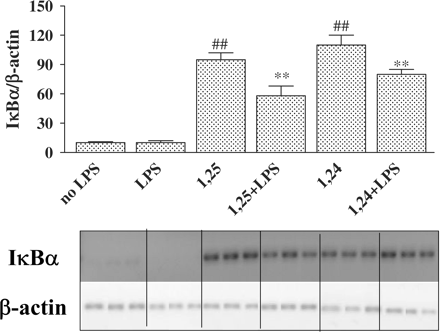
1,25(OH)2D3 and 1,24(OH)2D2 increase IκBα-mRNA levels.
To determine whether the induction of IκBα protein levels results from an increase in mRNA production, we analysed the effect of 1,25(OH)2D3 and 1,24(OH)2D2 on expression of IκBα-mRNA in P388D1 cells. RT-real-time PCR and RT-PCR were performed for IκBα-mRNA by using specific primers. As expected, the band for IκBα appeared at 469 bp (Figure 7). The densitometric evaluation expressed in Figure 7 indicates that IκBα-mRNA was significantly induced by 1,25(OH)2D3 and 1,24(OH)2D2. From Figure 7 we can speculate that one of the reasons for the increased expression of IκBα protein by 1,25(OH)2D3 and 1,24(OH)2D2 (Figure 6) is up-regulation of IκBα-mRNA levels.
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 increases IκBα-mRNA levels. Cells were incubated for 16 h with 1,25(OH)2D3 or 1,24(OH)2D2 (10−7 M) and then stimulated with LPS (1 µg/ml) for an additional 2.5 h. RT-PCR was performed on total cell RNA using specific primers for IκBα. PCR products (at 25 cycles) were separated on 2% agarose gel containing ethidium bromide (lower panel). The upper panel depicts a quantitative real-time RT-PCR of IκBα. IκBα-mRNA levels were normalized to β-actin levels. The results are expressed as mean±SE and represent three experiments performed in triplicate ##P<0.01 vs no LPS, **P<0.01 vs LPS alone.
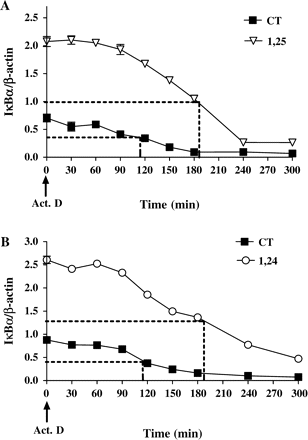
1,25(OH)2D3 and 1,24(OH)2D2 increase IκBα-mRNA stability.
The effect of 1,25(OH)2D3 and 1,24(OH)2D2 on IκBα-mRNA stability was examined by a real-time PCR technique, using actinomycin D, an inhibitor of DNA-depended RNA synthesis. Figure 8 shows that 1,25(OH)2D3 and 1,24(OH)2D2 inhibit IκBα-mRNA degradation. The half-life of IκBα-mRNA increased from 110 to 190 min in the presence of 1,25(OH)2D3 or 1,24(OH)2D2, indicating that IκBα-mRNA is more stable in P388D1 cells treated with 1,25(OH)2D3 or 1,24(OH)2D2 than in untreated cells (Figure 8A, B).
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 increases IκBα-mRNA stability. Cells were treated with 1,25(OH)2D3 and 1,24(OH)2D2 (10−7 M) for 16 h. Then actinomycin-D (1 µg/ml) was added, and cells were incubated for various lengths of time followed by RNA extraction. IκBα-mRNA levels were determined by real-time PCR. (A) 1,25(OH)2D3 and (B) 1,24(OH)2D2 inhibit the degradation of IκBα-mRNA. IκBα-mRNA levels were normalized by β-actin levels. Results for each panel are the mean±SE of one representative experiment out of three performed in triplicate.
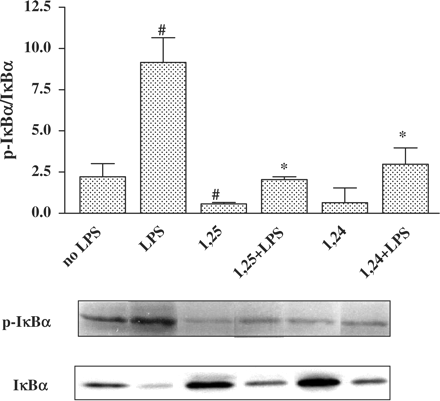
1,25(OH)2D3 and 1,24(OH)2D2 decrease IκBα phosphorylation.
1,25(OH)2D3 and 1,24(OH)2D2 augment the cellular content of IκBα protein (Figure 6) through an accumulation of IκBα-mRNA (Figure 7). Figure 8 shows that the induction of IκBα-mRNA caused by 1,25(OH)2D3 and 1,24(OH)2D2 results from increased IκBα-mRNA stability (Figure 8).
Numerous studies have reported a requirement for phosphorylation of IκBα on serine residues to target IκBα for ubiquitination and degradation. Therefore, another possible reason for the increase in IκBα protein levels by 1,25(OH)2D3 and 1,24(OH)2D2 may be inhibition of IκBα protein degradation. We next asked whether vitamin D affects the activity of IκB Kinase (IKK). IKK phosphorylates IκBα and targets its protein for ubiquitination and degradation by the 26S proteasome. The effect of vitamin D on the activity of IKK was examined by measuring the levels of phospho-IκBα at serine residues by western blot analysis, using an anti-phospho-IκBα antibody. Figure 9 shows that stimulation with LPS caused a slight increase in phosphorylation of IκBα protein while 1,25(OH)2D3 and 1,24(OH)2D2 induced a decrease in IκBα phosphorylation (Figure 9). Since phospho-IκBα levels are dependent on IKK-complex activity and on total IκBα levels, the values of phospho-IκBα were calculated for each treatment by the ratios of phospho-IκBα/total IκBα in order to compensate for differences in the amount of phospho-IκBα caused by its degradation (Figure 9).
Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 reduces phosphorylation of IκBα. Cells were treated with 1,25(OH)2D3 or 1,24(OH)2D2 for 16 h. This was followed by an additional 0.5 h of incubation with LPS (1 µg/ml). Cells were lysed and cytosol and total extracts of P388D1 cells were prepared, proteins were separated by SDS-PAGE and western blot analysis was carried out using an anti phospho−IκBα antibody. Qantitative evaluations of western blot analysis were performed by densitometric analysis, and the ratios of phospho-IκBα/IκBα were calculated. The results represent the mean±SE of three experiments #P<0.05 vs no LPS, *P<0.05 vs LPS alone.
Discussion
Similar to our findings from a previous investigation, [3,4], in our present study we showed that overnight incubation of peritoneal macrophages (16 h) with 1,25(OH)2D3 or 1,24(OH)2D2 significantly inhibited the secretion of TNFα in the murine macrophage cell line P388D1 in a dose-dependent manner. Given the methods we used in our experiments, activities such as cultivating large amounts of cells for western blot analysis or performing a transfection assay, were difficult to perform with human macrophages, and we were obliged to use a cell line. Since our findings showed that vitamin D had the same inhibitory effect on TNFα-mRNA and proteins levels in both peritoneal macrophages and P388D1 cells, we decided to use the P388D1 cells for our experiments. Following exposure to physiological levels of vitamin D (10−10 M), TNFα secretion was inhibited by 29%, this inhibition strengthening to as much as 61% at 10−7 M. Since peritoneal macrophages are able to metabolize 25-OH-D3 to its active metabolite 1,25(OH)2D3 [1], it is possible that in the microenvironment of the peritoneum the active concentrations of 1,25(OH)2D3 are higher than serum levels causing the inhibitory effect of vitamin D to be more profound.
The present study was aimed at exploring the mechanism underlying the inhibitory effect on TNFα and determining whether this effect is mediated by the activity of its major transcription factor, NFκB. We illustrated the inhibitory effect of 1,25(OH)2D3 and 1,24(OH)2D2 on LPS-induced NFκB activity by EMSA and by a promoter activity assay using luciferase as a reporter gene. Both assays clearly indicated that nuclei of vitamin D-treated cells stimulated with LPS contain less NFκB DNA-binding activity than untreated cells. We then tested whether the reduced LPS-induced NFκB activity was the result of a decrease in NFκB cellular content or of a rise in the cytosolic levels of its inhibitor IκBα. We found that vitamin D had no effect on total protein or mRNA levels of NFκB-p65 but did increase cytoplasmic NFκB-p65 levels and decreased NFκB-p65 levels in the nuclear portion of this transcription factor.
Inhibition of TNFα required a long incubation period with vitamin D suggesting that the inhibitory mechanism involves a synthesis or degradation of protein(s). Indeed the levels of IκBα which is responsible for retention of NFκB in the cytoplasm were upregulated in treated cells. 1,25(OH)2D3 and 1,24(OH)2D2 induced an increase of ∼6.5-fold in the levels of IκBα-mRNA and 4.5-fold in its protein levels. To elucidate the mechanism of IκBα upregulation, we tested whether the increase in IκBα levels is caused by changes in IκBα-mRNA stability and/or IκBα protein phosphorylation levels. IκBα-mRNA was found to be more stable in P388D1 cells treated with 1,25(OH)2D3 or 1,24(OH)2D2 (IκBα-mRNA half-life was prolonged from 110 to 190 min), indicating that these agents impede IκBα-mRNA degradation. We also found that vitamin D markedly reduced the basal and LPS-induced phosphorylation of IκBα. Since phosphorylation is the first step in marking IκBα for ubiquitination, the decrease in IκBα phosphorylation probably reduces its degradation. Hence, we can conclude that 1,25(OH)2D3 and 1,24(OH)2D2 upregulate IκBα levels through a dual mechanism, namely stabilization of mRNA and reduction of phosphorylation.
The main significance of our present investigation is that we succeeded in revealing a possible mechanism for the inhibition of TNFα by 1,25(OH)2D3 and 1,24(OH)2D2 in macrophages. This mechanism is mediated through NFκB, the major transcription factor of TNFα. NFκB regulates both the production of TNFα by mononuclear cells stimulated by LPS and the response of cells targeted by TNFα. To the best of our knowledge, this is the first time that the anti-inflammatory mechanism activity of the less calcaemic analog 1,24(OH)2D2 has been reported in the literature and raises its potentialities as an effective drug in combating inflammatory diseases.
Similar to our findings, other studies have shown that 1,25(OH)2D3 suppresses NFκB activity in a variety of cells. In melanoma cells, suppressed NFκB activity was associated with inhibition of IL-8 production [13], and in monocytic cells with decreased production of IL-12 and mRNA expression of IL-12 p35 and p40 subunits [14] and decreased production of chemokines by pancreatic islets [15]. In T-lymphocytes and peripheral blood mononuclear cells, the suppressive effect of 1,25(OH)2D3 on NFκB activity was associated with reduced cellular content of NFκB proteins. The suppressive effect of vitamin D on NFκB, therefore, would appear to be a general effect that occurs in various cell types and affects various inflammatory and immune processes. Furthermore, 1,25(OH)2D3 was demonstrated to suppress another transcription factor, NFATp/AP-1 which is involved in the biological activities of cytokines. 1,25(OH)2D3 represses IL-2 gene by a direct inhibition of VDR-dependent NFATp/AP-1 complex formation [16].
Similar to the effect of vitamin D, corticosteroids also decrease NFκB activity, at least partly, by up-regulating IκBα levels [17, 18]. In previous studies, we and others have shown that 1,25(OH)2D3 stimulates the killing capacity of macrophages, an effect mediated by an enhanced production of superoxide [19]. Other studies have also found that immunosuppression is caused by downregulation of the pro-inflammatory cytokine effector-TNFα by 1,25(OH)2D3. This diversity of anti-inflammatory effects of 1,25(OH)2D3 shows it to be an important immune response regulator at inflammatory sites. Recent reports on the successful use of 1,25(OH)2D3 as an anti-inflammatory agent in transplantations strengthen this hypothesis [20].
Although the present results suggest the therapeutic possibilities of 1,25(OH)2D3 as an anti-inflammatory agent, its clinical use is restricted because of its hypercalcaemic activity [2]. In previous in vitro studies we demonstrated the anti-inflammatory activity of vitamin D2 analog, 1,24(OH)2D2 and found it to be equipotential to that of 1,25(OH)2D3 [3, 4]. The present study confirmed that both agents are equipotential and share the same mechanism of NFκB inhibition. Since 1,24(OH)2D2 has a less calcaemic effect than 1,25(OH)2D3, its use as an anti-inflammatory agent may have clinical significance and deserves further investigation in order to validate our findings.
In conclusion, this study has demonstrated that 1,25(OH)2D3 and 1,24(OH)2D2 trigger the rise in IκBα protein and mRNA levels, as a result of stabilizing IκBα-mRNA and reducing IκBα phosphorylation. Elevated IκBα levels following overnight vitamin D treatment reduce the response to LPS by diminishing the amount of NFκB translocated to the nucleus. Since NFκB is needed for effective transcription of inflammatory genes, its reduced levels following vitamin D treatment is probably the cause of the inhibition of inflammatory genes such as TNFα.
This study was supported by a research grant provided by the Ministry of Health, Israel and by the ‘Dr Montague Robin Fleisher Kidney Transplant Unit Fund’.
Conflict of interest statement. None declared.
References
Shany S, Rapoport J, Zuili I, Gavriel A, Lavi N, Chaimovitz C. Metabolism of 25-OH-vitamin D3 by peritoneal macrophages from CAPD patients.
May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs.
Lahav-Cohen M, Douvdevani A, Chaimovitz C, Shany S. Regulation of TNF-alpha by 1alpha,25-dihydroxyvitamin D3 in human macrophages from CAPD patients.
Shany S, Levy Y, Lahav-Cohen M. The effects of 1alpha, 24(S)-dihydroxyvitamin D(2) analog on cancer cell proliferation and cytokine expression.
Schottelius AJ, Moldawer LL, Dinarello CA, Asadullah K, Sterry W, Edwards CK,3rd. Biology of tumor necrosis factor-alpha-implications for psoriasis.
Udalova IA, Knight JC, Vidal V, Nedospasov SA, Kwiatkowski D. Complex NF-kappaB interactions at the distal tumor necrosis factor promoter region in human monocytes.
Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors.
Liu H, Sidiropoulos P, Song G et al. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta.
Urban MB, Schreck R, Baeuerle PA. NF-kappa B contacts DNA by a heterodimer of the p50 and p65 subunit.
Jiang Y, Chen D, Lyu SC, Ling X, Krensky AM, Clayberger C. DQ 65–79, a peptide derived from HLA class II, induces I kappa B expression.
Jain RG, Phelps KD, Pekala PH. Tumor necrosis factor-alpha initiated signal transduction in 3T3-L1 adipocytes.
Harant H, Andrew PJ, Reddy GS, Foglar E, Lindley IJ. 1alpha,25-dihydroxyvitamin D3 and a variety of its natural metabolites transcriptionally repress nuclear-factor-kappaB-mediated interleukin-8 gene expression.
D’Ambrosio D, Cippitelli M, Cocciolo MG et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene.
Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development.
Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor.
Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS,Jr. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids.
Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis.
Deutsch A, Chaimovitzch C, Nagauker-Shriker O, Zlotnik M, Shany S, Levy R. Elevated superoxide generation in mononuclear phagocytes by treatment with 1 alpha hydroxyvitamin D3: changes in kinetics and in oxidase cytosolic factor p47.
Author notes
1Department of Clinical Biochemistry and 2Department of Nephrology, Soroka University Medical Center and Faculty of Health Sciences, Ben-Gurion University, PO Box 151, Beer-Sheva, 84101, Israel



![Treatment of P388D1 cells with 1,25(OH)2D3 or 1,24(OH)2D2 decreases NFκB DNA binding activity. Cells were incubated for 16 h with 1,25(OH)2D3 or 1,24(OH)2D2 (10−7 M) and then activated with LPS (1 µg/ml) for an additional 0.5 h. Nuclear extracts (20 µg protein/lane) were incubated with [32P]-end-labelled dsDNA oligonucleotide possessing the NFκB consensus sequence, and then the ability of bound proteins to induce retarded mobility of the radiolabelled probe on polyacrilamide gel was tested. Binding specificity was tested using 100-fold excess of ‘cold’ DNA probe and nuclear proteins from the nuclear extracts of activated cells. In addition, nuclear proteins from the nuclear extracts of activated cells were incubated with a competitive inhibitory antibody directed against p65 protein or with an isotype control antibody. One representative experiment out of five is shown.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/21/4/10.1093_ndt_gfi254/1/m_gfi254f3.gif?Expires=1716323010&Signature=Pjo1DFi9cSIsMCDWp3OtGdJtjjgKaqwssGJhfRS~Cs9DOlgn64rY2onLq2jHRpwhc6thf64JWYZj99X88sQcLW9y0HAy2ljjifFyN~qLovYRkqDnZ25w8wBDy8~ov~U7-e2zjRQwUfDJRX0cJ5uZnayBO8gHGSL0UOG6eMlcA1JdWSjU9f4xfHDQTnNW3HP4oYqZXOd7tIlrpJwGSL5TC9b5C32wdMBxpnHEB~PrI0WWtU9Fcq7bGlmMKmgJwjIn2CMy9NKgIVWg2z655SWkpDJXMjd4wnlU0r7t2O00k7nwUVBGdkVcMnJNVW13hO9IpL-wOCJbn4y6Aga4Z1xJMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


Comments