- 1Max Planck Institute for Heart and Lung Research, Member of the German Center for Lung Research (DZL), Member of the Cardio-Pulmonary Institute (CPI), Bad Nauheim, Germany
- 2Department of Internal Medicine, Member of the German Center for Lung Research (DZL), Member of the Cardio-Pulmonary Institute (CPI), Justus Liebig University, Giessen, Germany
- 3Frankfurt Cancer Institute (FCI), Goethe University, Frankfurt am Main, Germany
Regardless of the promising results of certain immune checkpoint blockers, current immunotherapeutics have met a bottleneck concerning response rate, toxicity, and resistance in lung cancer patients. Accumulating evidence forecasts that the crosstalk between tumor and immune cells takes center stage in cancer development by modulating tumor malignancy, immune cell infiltration, and immune evasion in the tumor microenvironment (TME). Cytokines and chemokines secreted by this crosstalk play a major role in cancer development, progression, and therapeutic management. An increased infiltration of Tumor-associated macrophages (TAMs) was observed in most of the human cancers, including lung cancer. In this review, we emphasize the role of cytokines and chemokines in TAM-tumor cell crosstalk in the lung TME. Given the role of cytokines and chemokines in immunomodulation, we propose that TAM-derived cytokines and chemokines govern the cancer-promoting immune responses in the TME and offer a new immunotherapeutic option for lung cancer treatment.
Introduction
Worldwide, Lung cancer is responsible for the highest number of cancer-related death in men and women (1). The 5-year survival rate in metastatic lung cancer (5%) is much lower than primary lung cancer (56%), colon (64.5%), breast (89.6%), and prostate (98.2%) cancer. Only 16% of lung cancer is diagnosed in early stages (2).
The first line of treatment in lung cancer is surgery, but most clinically detected cases are inoperable, and the chances of missing micro-metastasis and recurrence are high. When surgical intervention is not possible, then chemotherapy and radiotherapy are the next potential options, these therapies exert a devastating effect on normal tissue homeostasis and reduce health-related quality of life. An upcoming molecular targeted therapy targeting epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), improved treatment regimen in patients with these detectable mutations. Still, for a large group of lung cancers, molecular alterations have not been shown as effective for molecularly targeted therapies. Traditionally, immunotherapy showed marginal success in lung cancer. Recently, immune checkpoint blockers targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and anti-programmed cell death protein 1 (PD-1) have shown results in lung cancer treatment, but only a subset of the patients achieved a strong response with minimum toxicity in these immunotherapies (3–6), which is attributed to the fact that the lung tumor cells acquire large numbers of somatic mutations, and therefore induce tumor immune evasion by suppressing immune cell-mediated immunosurveillance via multiple mechanisms, such as secretion of pro-tumor cytokines, dysfunctional antigen expression, and inactivation of T cell activation (7–10). Thus, to develop new targeted therapies, future studies should be oriented toward the analysis of the tumor-infiltrating immune cells' landscape in the tumor microenvironment (TME) and how this contributes to lung carcinogenesis.
The driver mutations in tumors operate together and direct changes in the TME, especially in tumor-infiltrating immune cells (11). The driver mutations in lung cancer are gene mutations in EGFR and KRAS proto-oncogene (KRAS), ALK rearrangements, and altered MET proto-oncogene (MET) signaling (12). Notably, the extensive immunogenomic analysis of more than 10,000 samples from The Cancer Genome Atlas comprising 33 diverse cancer types displayed a more prominent macrophage signature with T helper (Th) 1 cell suppression and an M2-like macrophage response in tumors with gene mutations in EGFR, KRAS, and KRAS G12 (13). The infiltration of macrophages was found to be increased in lung tumor area when compared to the non-tumor area. Currently, there is no consensus in the literature on whether the high density of macrophages is detrimental or beneficial in lung cancer, while few recent reports demonstrated the correlation between prognosis and patient survival with the density of particular phenotype of macrophages. The prolonged patient survival correlated with a high density of M1 macrophages, while poor prognosis correlated with a high density of M2 macrophages in tumor islets (14–19). Evidences suggest that cytokines secreted by TAMs induce hyperproliferative, anti-apoptotic, and metastatic responses in lung cancer, offering a potential immunotherapeutic option for its treatment (20–22).
Tumor Associated Macrophages (TAMs)
Macrophages are a plastic, heterogeneous group of cells. Besides providing the innate immune response against invading pathogens, they play an essential role in maintaining tissue integrity and homeostasis. Macrophages encounter diverse microenvironmental signals, which can alter their transcriptional program and role, based on the location and distinct gene expression profiles. Functional and phenotypic dysregulation has been associated with a wide range of chronic inflammatory and autoimmune diseases, including cancer. Classically, macrophages divided into two major types, classical macrophage activation (M1) promotes a pro-inflammatory response, while alternative macrophage activation (M2) stimulates an anti-inflammatory response (23). Recently, according to specific cytokine stimulation conditions, M1 macrophages are subdivided into M (lipopolysaccharide [LPS]), M (LPS + interferon [IFN]γ), and M (IFNγ). M2 macrophages are subdivided into M (interleukin [IL] 4), M (immune complexes [Ic]), M(IL10), M (glucocorticoids [GC] + transforming growth factor β [TGFβ]), and M (GC) (24). These different types of macrophages drastically differ in their intrinsic transcription factors, metabolism, surface receptors, and secretory molecules, such as cytokines, chemokines, and growth factors, etc.
Tumor cell-macrophage crosstalk drives phenotypic and functional changes in both cell types. An intrinsic and extrinsic molecular patterning of tumor cells influences infiltration and activation of macrophages via multiple mechanisms: (i) Secretome of tumor cells shift the transcriptional program responsible for M1-like TAM activation to M2-like TAMs. Tumor cells derived Colony Stimulating Factor 1 (CSF1) and C-C motif chemokine ligand (CCL) 2 leads to increased infiltration of macrophages in TME, which later increased angiogenesis by stimulating the secretion of vascular endothelial growth factor (VEGF) (25). Tumor cells-macrophage co-culture increases expression of IL10, IL12, IL6, TNF, CCL5, CCL22, and CSF1 in macrophages, thereby inducing M2-like polarization (26). (ii) Apoptosis of tumor cells induces the activation of M2-like TAMs or suppress activation of M1-like TAMs (27). Apoptotic tumor cell-derived sphingosine-1-phosphate (S1P) and microRNA-375 alter macrophage polarization (28, 29). (iii) The alteration in macrophage function by necrotic tumor cells is still not very well elucidated. A study by Reiter et al. suggests that necrotic tumor cells promote the anti-tumor function of macrophages by increased production of nitric oxide (NO) (30). Another study by Brouckaert et al. suggests that phagocytosis of necrotic tumor cells by macrophages does not induce the production of inflammatory cytokines (31). Tumor cell-derived colony stimulating factor 1 (CSF1) promotes macrophage infiltration in the necrotic tumor area (32–34). These TAMs further support angiogenesis and invasion, and more interestingly, their high-density associated with reduced relapse-free survival (35, 36). (iv) Hypoxic tumor environments attract monocyte/macrophages followed by the differentiation and production of hypoxia-inducible factor (HIF) 1α and HIF2α, which then control the transcription of genes associated with tumor promotion processes, such as angiogenesis. Neuropilin 1 (NRP1) mediate hypoxic TME-induced activation and the pro-tumoral function of TAMs in cervical cancer (37). (v) The tumor cell-mediated metabolic shift in macrophage phenotype activates M2-like TAMs in TME. Through the mechanistic target of rapamycin kinase (mTOR) inhibition, TAMs from hypoxic areas show a decrease in glycolysis and increase in endothelial glucose availability, thereby disturbing a compact tumor vasculature to undergo invasion and metastasis (38). (vi) TAMs maintain an immunosuppressive phenotype by receiving polarization signals from tumor cells. IL1R and MYD88 mediated inhibitor of nuclear factor kappa B kinase subunit beta (IKBKB) and NFKB1 signaling cascade maintain M2-like phenotype in TAMs (39).
On the other hand, TAMs establish a pro-tumor microenvironment that influences the origin, progression, dissemination, and drug resistance of tumor cells in several ways, such as:(i) TAMs promote tumor cell growth and metastasis by secreting EGF (40), matrix metallopeptidase (MMP)s (41, 42), Wnt family member (WNT) 5A, cathepsin B, semaphorin 4D and IL1β (43). (ii) TAMs-derived migration inhibitory factor (MIF) induce DNA damage and immune escape by suppressing tumor protein P53 (TP53) activity (44). (iii) TAMs in hypoxic regions adapt to low oxygen tension by expressing HIF1α and subsequently secrete angiogenic factors, such as VEGF, IL8, cytochrome C oxidase assembly factor (COX2), and MMP9 (45). TAMs also increase tumor hypoxia and aerobic glycolysis (46). (iv) To support invasion and metastasis of tumor cells, TAMs induce epithelial-mesenchymal transition (EMT) in tumor cells via secretion of MMPs (47). (v) TAMs establish a pro-tumor anti-inflammatory environment by the recruitment of Th2 cells and regulatory T cells (48). (vi) TAMs play a part in T cell anergy and inhibition of the activation and growth of naïve T cells (49, 50). (vii) TAMs induced autocrine IL10 signaling pathway drives M2-like TAMs polarization to suppress anti-tumor response in TME (51). (viii) TAMs induce intrinsic activation of the immune checkpoint protein PDL1, which by binding to PD1 on T cells, leads to cytotoxic T cells senescence, exhaustion, and apoptosis (52).
Cytokines and Chemokines–Diagnostic and Prognostic Biomarkers in Lung Cancer
Although tumor cell-TAM crosstalk is dependent on many factors, secreted factors (such as cytokines, chemokines, etc.) play a significant role in the crosstalk. Cytokines and chemokines are low molecular weight proteins, mainly produced by macrophages and lymphocytes. They mediate intra- and extra-cellular communication as hormones and neurotransmitters through an autocrine, paracrine, and endocrine manner. Upon binding to specific cell surface receptors, they regulate a variety of cellular processes, such as local and systemic anti- and pro-inflammation, cellular proliferation, metabolism, chemotaxis, and tissue repair, etc. In the TME, the primary role of these factors is to regulate the tumor immunity cycle. Cytokines and chemokines produced by tumor-infiltrating immune cells play a significant role in tumor development, progression, metastasis, and therapy resistance; therefore, they widely used as diagnostic and prognostic biomarkers in the treatment of cancer. As shown in Table 1, most common cytokines and chemokines used in the therapeutic management of lung cancer are IL6, tumor necrosis factor α (TNFα), IL10, IFNγ, IL2, IL22, IL32, IL37, IL8, CCL2, C-X3-C motif chemokine ligand (CX3CL1) (53–59, 64, 82–85), among which macrophages are the major source of IL6, TNFα, IL10, IL8, CCL2, and CX3CL1 [(86, 87); Figure 1]. CCL2 and CX3CL1 receive special attention in chemokine biology, because of their unique phenotypic and functional properties. The decades of extensive research in the field of cytokines and chemokines in cancer development published outstanding research and review articles. Therefore, in this review, we summarized the published literature from the year 2000 to the year 2019, specifically focusing on the role of IL6, TNFα, IL10, CCL2, CX3CL1, IL8 in the macrophages-tumor cells crosstalk; leading to lung cancer development and progression.
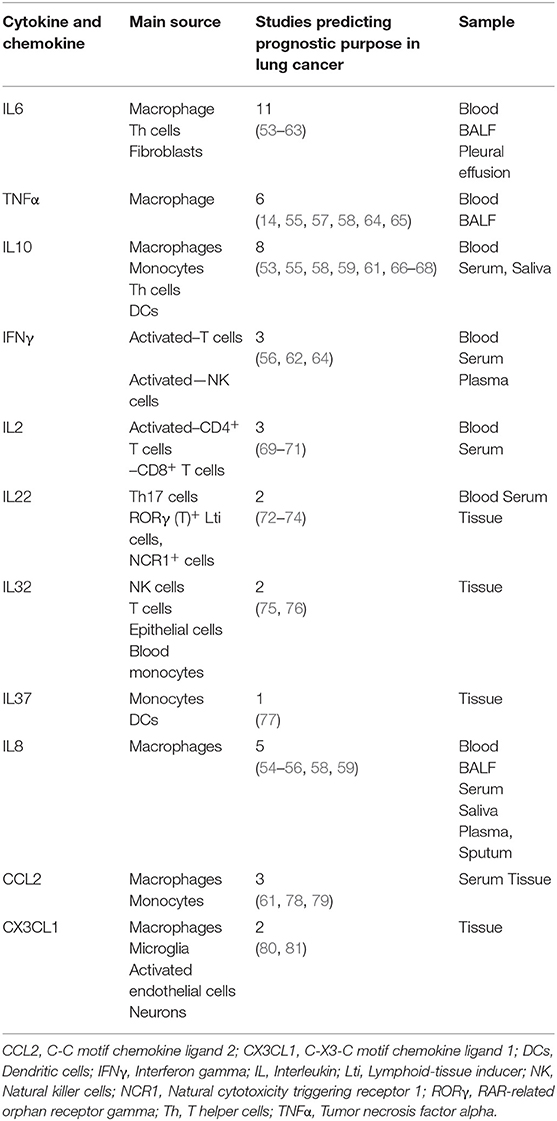
Table 1. Prognostic value of cytokines and chemokines in the therapeutic management of lung cancer and their main source of production.
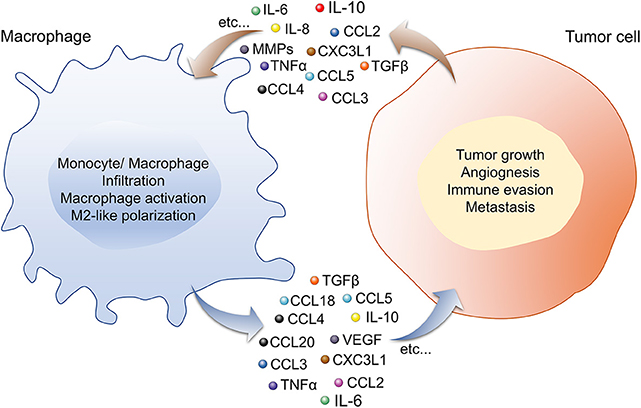
Figure 1. Macrophage-Tumor cells crosstalk via cytokines and chemokines through an autocrine and paracrine manner is important for lung cancer development. In the TME, cytokines, and chemokines secreted by macrophages (IL-6, -10, CCL-2, -3, -4, -5, -18, -20, CX3CL1, TGFβ, VEGF, TNFα, etc.) and tumor cells (IL-6, -8, -10, TNFα, CCL-2, -3, -4, -5, -18, -20, CX3CL1, MMPs, etc.) induce phenotypic and functional changes in both the cell types. Macrophage secretome influences tumor growth, angiogenesis, invasion, metastasis, and immune evasion by the tumor cell, while secretory factors from tumor cells regulate monocyte/macrophage infiltration, activation and polarization toward pro-tumor M2-like TAMs phenotype. Abbreviations: CCL, chemokine ligand; CXCL, chemokine (C-X-C motif) ligand; IL, interleukin; MMPs, matrix metalloproteinase; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Cytokines
IL6
IL6 plays a significant pro-inflammatory role under many physiological and pathological conditions in multiple cell types (88). Although many different cell types secrete IL6, macrophages are one of the major sources. IL-6 exerts its effects after binding to ligand-binding IL-6 receptor (IL6R) α chain (gp80, CD126) and the signal-transducing component gp130 (CD130) in an autocrine and paracrine manner. IL6 is a double-edged sword in the tumor microenvironment. Several studies demonstrated the role of IL6-mediated pro-proliferative, anti-apoptotic, angiogenic, metastatic, and immunosuppressive responses in tumor development and progression. While other studies demonstrated the role of IL6 in promoting anti-tumor immunity through the stimulation of proliferation, survival and trafficking of T cells to lymph nodes and tumor sites, where T cells effectively shift tumor-suppressive state to responsive state to inhibit tumor growth and progression (89, 90). An increased level of IL6 correlates with the poor prognosis and survival of lung cancer patients (54, 60). TAM-derived IL6 plays a role in progression, invasion, angiogenesis, EMT, immune cell infiltration, and cancer stem cell (CSC) development and maintenance, through multiple unexplored molecular mechanisms (91–93). The activation of signal transducer and activator of transcription (STAT) 3 by TAM-derived IL6 in the lung TME considered as the prime mechanism responsible for the development of mouse lung tumor model and crosstalk with small cell lung cancer (SCLC) cell lines (94, 95). Phosphatidylinositol-4,5-Bisphosphate 3-Kinase/AKT Serine/Threonine Kinase 1 (PI3K/AKT) signaling is another pathway engaged by TAM-derived IL6 to influence growth of lung cancer cell line, A549 (96). TAM-derived IL6-mediated STAT3 signaling pathway also found to increase the proliferation of human cancer stem cells (97). In different phases of lung cancer development and its therapeutic management, IL6 drives multiple molecular mechanisms responsible for the epithelial-mesenchymal transition (EMT) (98, 99) and therapy resistance, such as infiltration of pro-tumor macrophages after irradiation through the upregulation of CCL2/CCL5 in vitro human and in vivo mouse lung tumor models (100). Therefore, the blockade of IL6 reprograms the TME to restrict lung cancer development and progression in experimental lung tumorigenesis models (101). Many different approaches are used in various malignancies and other diseases to target IL-6 signaling pathways. For example–small molecules, blocking peptides, and antibodies against IL6, IL-6R, IL6–sIL6R complex, janus kinase (JAK) phosphorylation, and STAT3 activation (102, 103). The upregulation of systemic level of IL6 upon treatment of anti–PD1 antibody nivolumab leads to poor clinical outcome because inhibition of PD1–PDL1 promotes production of IL6 by PD1+ TAMs. Depletion of macrophages in vivo model of melanoma reduces the systemic level of IL6 and upregulates anti-tumor Th1 response, suggesting that the narrow therapeutic window of PD1–PDL1 blockade can be overcome by inhibition of IL6 (104).
TNFα
As the name suggests, TNFα initially found to induce necrosis and cytotoxicity in certain tumors (105). It is also known as a pyrogenic cytokine because of its ability to establish an inflammatory environment in response to pathogens (106). To exert a molecular action on the target cell, TNFα binds to one of the two receptors, TNF receptor superfamily member (TNFR1) (TNFRSF1A, p55TNFR1, p60, or CD120a) and TNFR2 (TNFRSF1B, p75TNFR, p80, or CD120b). According to the molecular context, TNFα exerts an opposite effect on tumor progression. In lung cancer, TNFα found to induce cell proliferation, apoptosis resistance, angiogenesis, invasion, and metastasis in various in vitro and in vivo lung tumor models (107). On the other hand, doxorubicin treatment-induced TNFα triggers apoptosis of TP53-deficient lung tumor cells via downregulation of cyclin dependent kinase inhibitor 1A (CDKN1A) (108). In the TME, crosstalk of TAMs with tumor cells and other tumor-associated cells via TNFα not only activates survival and proliferation pathways through the transcriptional activation of nuclear factor kappa B subunit 1 (NFKB1), fos proto-oncogene (FOS), and jun proto-oncogene (JUN) but also activates apoptotic pathways via TNFR1. Considering anti-tumor effects of TNFα, number of attempts were made to administer TNFα either systemically or locally in various cancer types. Although administration of TNFα significantly decreased the tumor growth, but many side effects were observed in the studies. In order to augment endogenous TNFα activity, Immunicon Inc. developed a single chain TNFα based affinity column to remove soluble TNF receptors from the blood (109). The pretreatment of low dose of TNFα prior to administration of chemotherapeutic agents such as Cisplatin, Paclitaxel, and Gemcitabine improved the efficacy of the agents in the experimental cancer model (110). On the hand recent studies showed that instead of augmenting effect of TNFα in tumor, TNFα blockade increases effect of immune checkpoint inhibitors (111, 112). Therefore, therapeutic approaches manipulating TNFα in cancer should be interpreted with great caution. The recent studies demonstrated that the higher number of tumor islets with infiltration of TNFα+ TAMs (cytotoxic M1 phenotype) confers a survival advantage in non-small-cell lung cancer (NSCLC) and other malignancies (14, 65). In TAMs-tumor cells in vitro co-culture model, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reprograms TAMs to M1-like phenotype by inducing expression of proinflammatory cytokines like IL1B, IL6, TNFα (113). TAMs-specific TNFα or its receptors induce apoptosis in vitro and in vivo tumor model by activating CD8+ T cells (114). Therefore, current immunotherapeutics need to be directed toward the induction of TNFα+ expression in TAMs, thereby reactivating anti-tumor immunity in the TME.
IL10
IL10 is an anti-inflammatory cytokine mainly produced by activated macrophages, B cells, and T cells (115). IL10 binds to the receptor IL10R. IL10R is a heterotetramer complex consisting of two IL10Rα and two IL10Rβ molecules (116). The main anti-inflammatory functions of IL10 are suppression of classical macrophage activation, suppression of the production of proinflammatory cytokines TNFα, IL1β, IL6, IL8, IL12, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (51, 117), inhibition of antigen presentation by suppressing major histocompatibility complex (MHC) II expression in activated macrophages (118), and inhibition of IFNγ production by Th1 and natural killer (NK) cells (119). Tumor cells often secrete a high amount of IL10, and increased serum concentration of IL10 found to be associated with simultaneous immunostimulation and immunosuppression in different types of cancer (120). The prognostic significance of IL10 in serum or whole tissue homogenate is controversial because a study by De vita et al. suggested that serum IL10 level was a prognostic indicator of advanced NSCLC (66) while studies by Soria et al. demonstrated that patients lacking IL10 expression in early-stage NSCLC have a worse prognosis than those with IL10 expression (67). On the other hand, TAMs-derived IL10 level showed consistent prognostic significance in lung cancer patients (121, 122). TAMs-derived IL10 perform several tasks in lung cancer progression and development (123). Similar to IL6, crosstalk within different cells of the TME via TAMs-derived IL10 leads to the activation of STAT3 (124, 125). Additionally, TAM-derived IL10 promotes the stemness of lung cancer via JAK1/STAT1/NFKB/NOTCH1 signaling pathways in vivo tumorigenesis mouse models (126). IL10 also drives in vivo lung cancer growth and metastasis by upregulating the CCL2/C-C motif chemokine receptor 2 (CCR2) and C-X-C motif chemokine ligand (CXC3CL1)/C-X3-C motif chemokine receptor 1 (CX3CR1) axis in macrophage-tumor cell crosstalk (20). IL10 signaling pathway is a complex molecular network comprising a minimum of 37 molecules and 76 reactions to support cancer development (127). The blockade of IL10 signaling pathways in human diseases is under critical investigation. Various strategies, such as blocking peptides and monoclonal antibodies against IL10, receptor-blocking strategies for IL10R, and small molecule inhibitors targeting JAK/STAT3 signaling, are under clinical evaluation (128, 129).
Chemokines
CCL2
Concerning the potential therapeutic intervention point in various human diseases, CCL2 is one of the most studied chemokines. CCL2 is a potent monocyte chemotactic factor from the C-C chemokine family. It mainly produced by monocyte/macrophages either constitutively or upon induction by other soluble factors and oxidative stress. CCL2 binds to its receptor CCR2 to mediate its effect through an autocrine or paracrine manner (130). Expression of CCL2, CCR2 or in combination with IL6 and IL10 correlates with a worse prognosis in lung cancer patients (61, 78, 79). In various cancers, the crosstalk of TAMs with tumor cells via the CCL2/CCR2 axis play multiple roles in cancer development, such as monocyte/macrophage recruitment at the tumor site (131), tumor progression, EMT, invasion, and metastasis (132). Experimental lung tumor models, in vitro TAMs-tumor cells, co-culture models, and human lung cancer biopsies demonstrated that CCL2/CCR2 signaling is one of the central signaling pathways involved in lung cancer growth and metastasis (20, 133, 134). In the in vivo metastasis lung tumor model and human lung cancer biopsies, infiltration of TAMs was found to be increased by NFKB1-CCL2 signaling via an elevation in neddylation pathway (135). In the flank and orthotopic lung tumor model, blockade of CCL2 reduces lung tumor growth not only by reprogramming TAMs to M1-like phenotype but also by activating CD8+ T cells (136). Deficiency of CCL2 in stromal cells like macrophages reduces infiltration of macrophages, angiogenesis, early tumor necrosis and lung metastasis in the 4T1 breast tumor model (137). Targeting of the CCL2/CCR2 axis in TAMs is an emerging immunotherapeutic tool in various human diseases. Therefore, therapeutic strategies blocking CCL2, CCR2, and CCL2/CCR2 complexes in TAMs are under extensive evaluation (138, 139).
CX3CL1
To date, CX3CL1 is the only known chemokine from the CX3C family. CXCL1 has received particular attention in chemokine biology because it is found in two forms, either bound to the cell membrane or in a soluble form. Therefore, it acts as both an adhesion molecule and chemoattractant (140). It plays a role in the activation and migration of monocytes, NK cells, T cells, and mast cells at the site of action in various physiological and pathological conditions. It also promotes the binding of leukocytes and the adhesion and activation of target cells. CX3CL1 mediates its cellular effects by interacting with CX3CR1 (141). A recent study by Liu et al. demonstrated that high CX3CL1 mRNA expression served as a positive prognostic indicator in patients with lung adenocarcinoma (80). Another study by Su et al. demonstrated that the CXC3CL1 level showed survival effects in lung adenocarcinoma but not in squamous cell carcinoma (81). The expression of CX3CL1 was found to be increased in lung cancer with higher pathological grades and metastatic lymph nodes (142). CX3CL1-induced M2 macrophage polarization increases invasiveness of human endometrial stromal cells (ESCs) by upregulating expression of MMP9, tissue inhibitor of metalloproteinases (TIMP)-1, -2 and by activating P38MAPK and integrinβ1 signaling (143). In the co-culture of peripheral blood mononuclear cells (PBMCs) and pancreatic cancer cell lines, TRAIL/NFKB1/CX3CL1 dependant bi-directional crosstalk leads to therapy resistance (144). In the mouse mammary tumor model, activation of fibroblast growth factor receptor 1 (FGFR1) leads to migration and recruitment of macrophages via secretion of CX3CL1 (145). In in vitro, in vivo, and ex vivo models of lung cancer, TAM-tumor cell crosstalk via the CX3CL1/CX3CR1 axis found to be crucial in lung tumor cell growth, and metastasis, suggesting a potential axis for therapeutic intervention in lung cancer (20).
IL8
IL8/CXCL8 is a proinflammatory chemokine (from the CXC family) mainly secreted by macrophages. It exerts its effect by binding to CXCR1 and CXCR2, which are heterotrimeric G-protein-coupled receptors. These receptors are expressed not only by neutrophils but also by monocytes, endothelial cells, tumor cells, and tumor-associated stromal cells (146). Therefore, IL-8 is responsible for the migration and activation of all these cells (87). IL-8 plays multiple roles in lung cancer development (147–151). IL8 serves as a potential biomarker to predict tumor burden, treatment response, and patient survival in lung cancer (152–154). The expression of IL8 mRNA in the lung TME induced by infiltrating macrophages via the NFKB pathway significantly correlates with increased tumor angiogenesis and shorter median survival of lung cancer (155). IL8 is known to activate major oncogenic signaling pathways through autocrine and paracrine functions in the TME [e.g., PI3K, RAS/mitogen-activated protein kinase (MAPK), and JAK/STAT] (156). Therefore, the precise molecular role of TAM-derived IL8 in TAMs-tumor cell crosstalk requires further investigation. To block IL8 dependent responses, IL8 neutralizing antibodies (ABX-IL8 and HuMax-IL8), small molecule inhibitor of CX3CR1/CX3CR2 (Reparixin, JMS-17-2) are in the different phases of preclinical and clinical development (157, 158).
Conclusion
The understanding of cytokines and chemokines-mediated interplay between TAMs and tumor cells on a molecular level will allow the development of new immunotherapeutic strategies aimed to unleash the anti-tumor immunity of TAMs in the TME. Given the potential immunomodulatory role of TAM-specific secretory factors in lung cancer development and progression, it is crucial to address the following questions to develop a therapeutic strategy: (i) Are the pro- and anti-inflammation environments triggered by TAM-derived cytokines and chemokines context-dependent? (ii) How does cytokine- and chemokine-based TAM-tumor cell crosstalk influence other immune cells of the TME? (iii) Which are the central signaling pathways regulated by TAM-derived cytokines and chemokines that influence cancer development? A critical evaluation of therapeutic strategies targeting cytokines and chemokines is required to develop potent and efficient immunotherapeutics to restrict cancer development and further validation of these experimental findings in patient samples required for clinical translation.
Author Contributions
PS, MS, FG, WS, and RS contributed to the conception, writing, and editing for this manuscript.
Funding
This work was supported by the Max Planck Society, Verein zur Förderung der Krebsforschung in Gieβen e.V., Von-Behring-Röntgen-Stiftung, a Rhön Klinikum AG grant, Frankfurt Cancer Institute (LOEWE FCI), Cardio-Pulmonary Institute (CPI), and the German Center for Lung Research (DZL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
3. Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. (2003) 100:4712–7. doi: 10.1073/pnas.0830997100
4. Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. (2003) 100:8372–7. doi: 10.1073/pnas.1533209100
5. Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer. (2018) 6:39. doi: 10.1186/s40425-018-0349-3
6. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. (2018) 8:86. doi: 10.3389/fonc.2018.00086
7. Massarelli E, Papadimitrakopoulou V, Welsh J, Tang C, Tsao AS. Immunotherapy in lung cancer. Transl Lung Cancer Res. (2014) 3:53–63. doi: 10.3978/j.issn.2218-6751.2014.01.01
8. Yang L, Wang L, Zhang Y. Immunotherapy for lung cancer: advances and prospects. Am J Clin Exp Immunol. (2016) 5:1–20.
9. Corrales L, Scilla K, Caglevic C, Miller K, Oliveira J, Rolfo C. Immunotherapy in lung cancer: a new age in cancer treatment. Adv Exp Med Biol. (2018) 995:65–95. doi: 10.1007/978-3-030-02505-2_3
10. Mascaux C, Angelova M, Vasaturo A, Beane J, Hijazi K, Anthoine G, et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. (2019) 571:570–5. doi: 10.1038/s41586-019-1330-0
11. Seton-Rogers S. Oncogenes: driving immune evasion. Nat Rev Cancer. (2018) 18:67. doi: 10.1038/nrc.2018.5
12. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. (2018) 173:321–337.e10. doi: 10.1016/j.cell.2018.03.035
13. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. (2018) 48:812–830.e14. doi: 10.1016/j.immuni.2018.03.023
14. Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. (2009) 33:118–26. doi: 10.1183/09031936.00065708
15. Banat GA, Tretyn A, Pullamsetti SS, Wilhelm J, Weigert A, Olesch C, et al. Immune and inflammatory cell composition of human lung cancer stroma. PLoS ONE. (2015) 10:e0139073. doi: 10.1371/journal.pone.0139073
16. Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. (2018) 17:887–904. doi: 10.1038/nrd.2018.169
17. Cao L, Che X, Qiu X, Li Z, Yang B, Wang S, et al. M2 macrophage infiltration into tumor islets leads to poor prognosis in non-small-cell lung cancer. Cancer Manag Res. (2019) 11:6125–38. doi: 10.2147/CMAR.S199832
18. Rakaee M, Busund LR, Jamaly S, Paulsen EE, Richardsen E, Andersen S, et al. Prognostic value of macrophage phenotypes in resectable non-small cell lung cancer assessed by multiplex immunohistochemistry. Neoplasia. (2019) 21:282–93. doi: 10.1016/j.neo.2019.01.005
19. Stankovic B, Bjørhovde HAK, Skarshaug R, Aamodt H, Frafjord A, Müller E, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol. (2019) 9:3101–3101. doi: 10.3389/fimmu.2018.03101
20. Schmall A, Al-Tamari HM, Herold S, Kampschulte M, Weigert A, Wietelmann A, et al. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med. (2015) 191:437–47. doi: 10.1164/rccm.201406-1137OC
21. Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. (2017) 195:425–37. doi: 10.1164/rccm.201606-1226PP
22. Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1beta. J Exp Med. (2017) 214:2695–713. doi: 10.1084/jem.20160392
23. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. (2000) 164:6166–73. doi: 10.4049/jimmunol.164.12.6166
24. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
25. Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. (2007) 67:5064–6. doi: 10.1158/0008-5472.CAN-07-0912
26. Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. (2006) 176:5023–32. doi: 10.4049/jimmunol.176.8.5023
27. Voss JJLP, Ford CA, Petrova S, Melville L, Paterson M, Pound JD, et al. Modulation of macrophage antitumor potential by apoptotic lymphoma cells. Cell Death Differ. (2017) 24:971–83. doi: 10.1038/cdd.2016.132
28. Weigert A, Tzieply N, von Knethen A, Johann AM, Schmidt H, Geisslinger G, et al. Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol Biol Cell. (2007) 18:3810–9. doi: 10.1091/mbc.e06-12-1096
29. Frank AC, Ebersberger S, Fink AF, Lampe S, Weigert A, Schmid T, et al. Apoptotic tumor cell-derived microRNA-375 uses CD36 to alter the tumor-associated macrophage phenotype. Nat Commun. (2019) 10:1135. doi: 10.1038/s41467-019-08989-2
30. Reiter I, Krammer B, Schwamberger G. Cutting edge: differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol. (1999) 163:1730–2.
31. Brouckaert G, Kalai M, Krysko DV, Saelens X, Vercammen D, Ndlovu MN, et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell. (2004) 15:1089–100. doi: 10.1091/mbc.e03-09-0668
32. Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. (2001) 193:727–40. doi: 10.1084/jem.193.6.727
33. Esserman LJ, Kumar AS, Herrera AF, Leung J, Au A, Chen Y-Y, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol. (2006) 24:4603–10. doi: 10.1200/JCO.2005.04.5518
34. Sharma M, Beck AH, Webster JA, Espinosa I, Montgomery K, Varma S, et al. Analysis of stromal signatures in the tumor microenvironment of ductal carcinoma in situ. Breast Cancer Res Treat. (2010) 123:397–404. doi: 10.1007/s10549-009-0654-0
35. Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. (1999) 79:991–5. doi: 10.1038/sj.bjc.6690158
36. Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, et al. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. (2004) 24:3335–42.
37. Chen XJ, Wu S, Yan RM, Fan LS, Yu L, Zhang YM, et al. The role of the hypoxia-Nrp-1 axis in the activation of M2-like tumor-associated macrophages in the tumor microenvironment of cervical cancer. Mol Carcinog. (2019) 58:388–97. doi: 10.1002/mc.22936
38. Wenes M, Shang M, Di Matteo M, Goveia J, Martin-Perez R, Serneels J, et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. (2016) 24:701–15. doi: 10.1016/j.cmet.2016.09.008
39. Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. (2008) 205:1261–8. doi: 10.1084/jem.20080108
40. Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. (2005) 65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853
41. Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. (2004) 25:1543–9. doi: 10.1093/carcin/bgh146
42. Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. (2005) 7:485–96. doi: 10.1016/j.ccr.2005.04.013
43. Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. (2009) 1796:11–8. doi: 10.1016/j.bbcan.2009.02.004
44. Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. (1999) 190:1375–82. doi: 10.1084/jem.190.10.1375
45. Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. (2006) 2:213–9. doi: 10.2147/vhrm.2006.2.3.213
46. Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE, Bok S, et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res. (2019) 79:795–806. doi: 10.1158/0008-5472.CAN-18-2545
47. Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, et al. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer. (2011) 74:188–96. doi: 10.1016/j.lungcan.2011.04.009
48. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
49. Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. (2004) 64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465
50. Johnson TS, Munn DH. Host indoleamine 2,3-dioxygenase: contribution to systemic acquired tumor tolerance. Immunol Invest. (2012) 41:765–97. doi: 10.3109/08820139.2012.689405
51. Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. (2000) 164:762–7. doi: 10.4049/jimmunol.164.2.762
52. Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. (2009) 206:1327–37. doi: 10.1084/jem.20082173
53. Matanic D, Beg-Zec Z, Stojanovic D, Matakoric N, Flego V, Milevoj-Ribic F. Cytokines in patients with lung cancer. Scand J Immunol. (2003) 57:173–8. doi: 10.1046/j.1365-3083.2003.01205.x
54. Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. (2011) 103:1112–22. doi: 10.1093/jnci/djr216
55. Song XY, Zhou SJ, Xiao N, Li YS, Zhen DZ, Su CY, et al. Research on the relationship between serum levels of inflammatory cytokines and non-small cell lung cancer. Asian Pac J Cancer Prev. (2013) 14:4765–8. doi: 10.7314/APJCP.2013.14.8.4765
56. Barrera L, Montes-Servin E, Barrera A, Ramirez-Tirado LA, Salinas-Parra F, Banales-Mendez JL, et al. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Ann Oncol. (2015) 26:428–35. doi: 10.1093/annonc/mdu549
57. DeCotiis C, Hu Y, Greenberg AK, Huie M, Tsay JC, Pass H, et al. Inflammatory cytokines and non-small cell lung cancer in a CT-scan screening cohort: Background review of the literature. Cancer Biomark. (2016) 16:219–33. doi: 10.3233/CBM-150559
58. Brenner DR, Fanidi A, Grankvist K, Muller DC, Brennan P, Manjer J, et al. Inflammatory cytokines and lung cancer risk in 3 prospective studies. Am J Epidemiol. (2017) 185:86–95. doi: 10.1093/aje/kww159
59. Koizumi T, Shetty V, Yamaguchi M. Salivary cytokine panel indicative of non-small cell lung cancer. J Int Med Res. (2018) 46:3570–82. doi: 10.1177/0300060518775563
60. Silva EM, Mariano VS, Pastrez PRA, Pinto MC, Castro AG, Syrjanen KJ, et al. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS ONE. (2017) 12:e0181125. doi: 10.1371/journal.pone.0181125
61. Pan YW, Zhou ZG, Wang M, Dong JQ, Du KP, Li S, et al. Combination of IL-6, IL-10, and MCP-1 with traditional serum tumor markers in lung cancer diagnosis and prognosis. Genet Mol Res. (2016) 15. doi: 10.4238/gmr15048949
62. Martín F, Santolaria F, Batista N, Milena A, González-Reimers E, Brito MJ, et al. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. (1999) 11:80–6. doi: 10.1006/cyto.1998.0398
63. Crohns M, Saarelainen S, Laine S, Poussa T, Alho H, Kellokumpu-Lehtinen P. Cytokines in bronchoalveolar lavage fluid and serum of lung cancer patients during radiotherapy - Association of interleukin-8 and VEGF with survival. Cytokine. (2010) 50:30–6. doi: 10.1016/j.cyto.2009.11.017
64. Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G, et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. (2009) 18:215–22. doi: 10.1158/1055-9965.EPI-08-0705
65. Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer. (2010) 10:323. doi: 10.1186/1471-2407-10-323
66. De Vita F, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E, et al. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest. (2000) 117:365–73. doi: 10.1378/chest.117.2.365
67. Soria JC, Moon C, Kemp BL, Liu DD, Feng L, Tang X, et al. Lack of interleukin-10 expression could predict poor outcome in patients with stage I non-small cell lung cancer. Clin Cancer Res. (2003) 9:1785–91.
68. Hatanaka H, Abe Y, Kamiya T, Morino F, Nagata J, Tokunaga T, et al. Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Ann Oncol. (2000) 11:815–9. doi: 10.1023/A:1008375208574
69. Fischer JR, Schindel M, Bulzebruck H, Lahm H, Krammer PH, Drings P. Long-term survival in small cell lung cancer patients is correlated with high interleukin-2 secretion at diagnosis. J Cancer Res Clin Oncol. (2000) 126:730–3. doi: 10.1007/PL00008479
70. Orditura M, Romano C, De Vita F, Galizia G, Lieto E, Infusino S, et al. Behaviour of interleukin-2 serum levels in advanced non-small-cell lung cancer patients: relationship with response to therapy and survival. Cancer Immunol Immunother. (2000) 49:530–6. doi: 10.1007/s002620000150
71. Yan X, Jiao SC, Zhang GQ, Guan Y, Wang JL. Tumor-associated immune factors are associated with recurrence and metastasis in non-small cell lung cancer. Cancer Gene Therapy. (2017) 24:57–63. doi: 10.1038/cgt.2016.40
72. Kobold S, Volk S, Clauditz T, Kupper NJ, Minner S, Tufman A, et al. Interleukin-22 is frequently expressed in small- and large-cell lung cancer and promotes growth in chemotherapy-resistant cancer cells. J Thorac Oncol. (2013) 8:1032–42. doi: 10.1097/JTO.0b013e31829923c8
73. Guillon A, Gueugnon F, Mavridis K, Dalloneau E, Jouan Y, Diot P, et al. Interleukin-22 receptor is overexpressed in nonsmall cell lung cancer and portends a poor prognosis. Eur Respir J. (2016) 47:1277–80. doi: 10.1183/13993003.01580-2015
74. Khosravi N, Caetano MS, Cumpian AM, Unver N, De la Garza Ramos C, Noble O, et al. IL22 promotes Kras-mutant lung cancer by induction of a protumor immune response and protection of stemness properties. Cancer Immunol Res. (2018) 6:788–97. doi: 10.1158/2326-6066.CIR-17-0655
75. Sorrentino C, Di Carlo E. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med. (2009) 180:769–79. doi: 10.1164/rccm.200903-0400OC
76. Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang L, et al. Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine. (2014) 65:24–32. doi: 10.1016/j.cyto.2013.09.017
77. Ge G, Wang A, Yang J, Chen Y, Yang J, Li Y, et al. Interleukin-37 suppresses tumor growth through inhibition of angiogenesis in non-small cell lung cancer. J Exp Clin Cancer Res. (2016) 35:13. doi: 10.1186/s13046-016-0293-3
78. Zhang XW, Qin X, Qin CY, Yin YL, Chen Y, Zhu HL. Expression of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in non-small cell lung cancer and its significance. Cancer Immunol Immunother. (2013) 62:563–70. doi: 10.1007/s00262-012-1361-y
79. Li L, Liu YD, Zhan YT, Zhu YH, Li Y, Xie D, et al. High levels of CCL2 or CCL4 in the tumor microenvironment predict unfavorable survival in lung adenocarcinoma. Thorac Cancer. (2018) 9:775–84. doi: 10.1111/1759-7714.12643
80. Liu J, Li Y, Zhu X, Li Q, Liang X, Xie J, et al. Increased CX3CL1 mRNA expression level is a positive prognostic factor in patients with lung adenocarcinoma. Oncol Lett. (2019) 17:4877–90. doi: 10.3892/ol.2019.10211
81. Su YC, Chang H, Sun SJ, Liao CY, Wang LY, Ko JL, et al. Differential impact of CX3CL1 on lung cancer prognosis in smokers and non-smokers. Mol Carcinog. (2018) 57:629–39. doi: 10.1002/mc.22787
82. Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS ONE. (2011) 6:e17479. doi: 10.1371/journal.pone.0017479
83. Hofmann JN, Yu K, Bagni RK, Lan Q, Rothman N, Purdue MP. Intra-individual variability over time in serum cytokine levels among participants in the prostate, lung, colorectal, and ovarian cancer screening trial. Cytokine. (2011) 56:145–8. doi: 10.1016/j.cyto.2011.06.012
84. Marrugal A, Ojeda L, Paz-Ares L, Molina-Pinelo S, Ferrer I. Proteomic-based approaches for the study of cytokines in lung cancer. Dis Markers. (2016) 2016:2138627. doi: 10.1155/2016/2138627
85. Misra P, Singh S. Role of cytokines in combinatorial immunotherapeutics of non-small cell lung cancer through systems perspective. Cancer Med. (2019) 8:1976–95. doi: 10.1002/cam4.2112
86. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. (2014) 5:491. doi: 10.3389/fimmu.2014.00491
87. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. (2014) 1843:2563–82. doi: 10.1016/j.bbamcr.2014.05.014
88. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. (2011) 1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034
89. Haabeth OA, Bogen B, Corthay A. A model for cancer-suppressive inflammation. Oncoimmunology. (2012) 1:1146–55. doi: 10.4161/onci.21542
90. Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. (2014) 26:38–47. doi: 10.1016/j.smim.2014.01.008
91. Dehai C, Bo P, Qiang T, Lihua S, Fang L, Shi J, et al. Enhanced invasion of lung adenocarcinoma cells after co-culture with THP-1-derived macrophages via the induction of EMT by IL-6. Immunol Lett. (2014) 160:1–10. doi: 10.1016/j.imlet.2014.03.004
92. Gomes M, Coelho A, Araujo A, Azevedo A, Teixeira AL, Catarino R, et al. IL-6 polymorphism in non-small cell lung cancer: a prognostic value? Tumour Biol. (2015) 36:3679–84. doi: 10.1007/s13277-014-3006-6
93. Qu Z, Sun F, Zhou J, Li L, Shapiro SD, Xiao G. Interleukin-6 prevents the initiation but enhances the progression of lung cancer. Cancer Res. (2015) 75:3209–15. doi: 10.1158/0008-5472.CAN-14-3042
94. Iriki T, Ohnishi K, Fujiwara Y, Horlad H, Saito Y, Pan C, et al. The cell-cell interaction between tumor-associated macrophages and small cell lung cancer cells is involved in tumor progression via STAT3 activation. Lung Cancer. (2017) 106:22–32. doi: 10.1016/j.lungcan.2017.01.003
95. Unver N, Delgado O, Zeleke K, Cumpian A, Tang X, Caetano MS, et al. Reduced IL-6 levels and tumor-associated phospho-STAT3 are associated with reduced tumor development in a mouse model of lung cancer chemoprevention with myo-inositol. Int J Cancer. (2018) 142:1405–17. doi: 10.1002/ijc.31152
96. Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning W, et al. PD-L1 induced by IFN-gamma from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int J Clin Oncol. (2017) 22:1026–33. doi: 10.1007/s10147-017-1161-7
97. Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. (2014) 147:1393–404. doi: 10.1053/j.gastro.2014.08.039
98. Ogawa H, Koyanagi-Aoi M, Otani K, Zen Y, Maniwa Y, Aoi T. Interleukin-6 blockade attenuates lung cancer tissue construction integrated by cancer stem cells. Sci Rep. (2017) 7:12317. doi: 10.1038/s41598-017-12017-y
99. Chen Y, Tan W, Wang C. Tumor-associated macrophage-derived cytokines enhance cancer stem-like characteristics through epithelial-mesenchymal transition. Onco Targets Ther. (2018) 11:3817–26. doi: 10.2147/OTT.S168317
100. Wang X, Yang X, Tsai Y, Yang L, Chuang KH, Keng PC, et al. IL-6 Mediates macrophage infiltration after irradiation via Up-regulation of CCL2/CCL5 in non-small cell lung cancer. Radiat Res. (2017) 187:50–9. doi: 10.1667/RR14503.1
101. Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, et al. IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras-mutant lung cancer. Cancer Res. (2016) 76:3189–99. doi: 10.1158/0008-5472.CAN-15-2840
102. Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. (2018) 15:234–48. doi: 10.1038/nrclinonc.2018.8
103. Pullamsetti SS, Seeger W, Savai R. Classical IL-6 signaling: a promising therapeutic target for pulmonary arterial hypertension. J Clin Invest. (2018) 128:1720–3. doi: 10.1172/JCI120415
104. Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. (2018) 78:5011–22. doi: 10.1158/0008-5472.CAN-18-0118
105. Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. (1975) 72:3666–70. doi: 10.1073/pnas.72.9.3666
106. Vinet AF, Fukuda M, Descoteaux A. The exocytosis regulator synaptotagmin V controls phagocytosis in macrophages. J Immunol. (2008) 181:5289–95. doi: 10.4049/jimmunol.181.8.5289
107. Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. (2008) 29:1275–88. doi: 10.1111/j.1745-7254.2008.00889.x
108. Cao W, Chi WH, Wang J, Tang JJ, Lu YJ. TNF-alpha promotes Doxorubicin-induced cell apoptosis and anti-cancer effect through downregulation of p21 in p53-deficient tumor cells. Biochem Biophys Res Commun. (2005) 330:1034–40. doi: 10.1016/j.bbrc.2005.02.188
109. Josephs SF, Ichim TE, Prince SM, Kesari S, Marincola FM, Escobedo AR, et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med. (2018) 16:242. doi: 10.1186/s12967-018-1611-7
110. Sacchi A, Gasparri A, Gallo-Stampino C, Toma S, Curnis F, Corti A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin Cancer Res. (2006) 12:175–82. doi: 10.1158/1078-0432.CCR-05-1147
111. Bertrand F, Montfort A, Marcheteau E, Imbert C, Gilhodes J, Filleron T, et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. (2017) 8:2256–2256. doi: 10.1038/s41467-017-02358-7
112. Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. (2019) 569:428–32. doi: 10.1038/s41586-019-1162-y
113. Gao J, Wang D, Liu D, Liu M, Ge Y, Jiang M, et al. Tumor necrosis factor-related apoptosis-inducing ligand induces the expression of proinflammatory cytokines in macrophages and re-educates tumor-associated macrophages to an antitumor phenotype. Mol Biol Cell. (2015) 26:3178–89. doi: 10.1091/mbc.e15-04-0209
114. Saio M, Radoja S, Marino M, Frey AB. Tumor-infiltrating macrophages induce apoptosis in activated CD8(+) T cells by a mechanism requiring cell contact and mediated by both the cell-associated form of TNF and nitric oxide. J Immunol. (2001) 167:5583–93. doi: 10.4049/jimmunol.167.10.5583
115. Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. (2008) 226:205–18. doi: 10.1111/j.1600-065X.2008.00706.x
116. Walter MR. The molecular basis of IL-10 function: from receptor structure to the onset of signaling. Curr Top Microbiol Immunol. (2014) 380:191–212. doi: 10.1007/978-3-662-43492-5_9
117. Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. (1991) 147:3815–22.
118. Chadban SJ, Tesch GH, Foti R, Lan HY, Atkins RC, Nikolic-Paterson DJ. Interleukin-10 differentially modulates MHC class II expression by mesangial cells and macrophages in vitro and in vivo. Immunology. (1998) 94:72–8. doi: 10.1046/j.1365-2567.1998.00487.x
119. Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. (1992) 175:671–82. doi: 10.1084/jem.175.3.671
120. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. (2013) 14:e218–28. doi: 10.1016/S1470-2045(12)70582-X
121. Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, Querzoli P, et al. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J. (2007) 30:627–32. doi: 10.1183/09031936.00129306
122. Wang R, Lu M, Zhang J, Chen S, Luo X, Qin Y, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res. (2011) 30:62. doi: 10.1186/1756-9966-30-62
123. Montuenga LM, Pio R. Tumour-associated macrophages in nonsmall cell lung cancer: the role of interleukin-10. Eur Respir J. (2007) 30:608–10. doi: 10.1183/09031936.00091707
124. Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. (2007) 178:2623–9. doi: 10.4049/jimmunol.178.5.2623
125. Chen L, Shi Y, Zhu X, Guo W, Zhang M, Che Y, et al. IL10 secreted by cancerassociated macrophages regulates proliferation and invasion in gastric cancer cells via cMet/STAT3 signaling. Oncol Rep. (2019) 42:595–604. doi: 10.3892/or.2019.7206
126. Yang L, Dong Y, Li Y, Wang D, Liu S, Wang D, et al. IL-10 derived from M2 macrophage promotes cancer stemness via JAK1/STAT1/NF-kappaB/Notch1 pathway in non-small cell lung cancer. Int J Cancer. (2019) 145:1099–110. doi: 10.1002/ijc.32151
127. Verma R, Balakrishnan L, Sharma K, Khan AA, Advani J, Gowda H, et al. A network map of Interleukin-10 signaling pathway. J Cell Commun Signal. (2016) 10:61–7. doi: 10.1007/s12079-015-0302-x
128. Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. (2014) 26:623–37. doi: 10.1016/j.ccell.2014.09.006
129. Wang X, Wong K, Ouyang W, Rutz S. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol. (2019) 11:a028548. doi: 10.1101/cshperspect.a028548
130. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. (2009) 29:313–26. doi: 10.1089/jir.2008.0027
131. Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. (2011) 475:222–5. doi: 10.1038/nature10138
132. Tang CH, Tsai CC. CCL2 increases MMP-9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF-kappaB signaling pathway. Biochem Pharmacol. (2012) 83:335–44. doi: 10.1016/j.bcp.2011.11.013
133. Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. (2016) 7:28697–710. doi: 10.18632/oncotarget.7376
134. Ding M, He SJ, Yang J. MCP-1/CCL2 Mediated by autocrine loop of PDGF-BB promotes invasion of lung cancer cell by recruitment of macrophages via CCL2-CCR2 axis. J Interferon Cytokine Res. (2019) 39:224–32. doi: 10.1089/jir.2018.0113
135. Zhou L, Jiang Y, Liu X, Li L, Yang X, Dong C, et al. Promotion of tumor-associated macrophages infiltration by elevated neddylation pathway via NF-κB-CCL2 signaling in lung cancer. Oncogene. (2019) 38:5792–804. doi: 10.1038/s41388-019-0840-4
136. Fridlender ZG, Kapoor V, Buchlis G, Cheng G, Sun J, Wang LC, et al. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am J Respir Cell Mol Biol. (2011) 44:230–7. doi: 10.1165/rcmb.2010-0080OC
137. Yoshimura T, Howard OM, Ito T, Kuwabara M, Matsukawa A, Chen K, et al. Monocyte chemoattractant protein-1/CCL2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells. PLoS ONE. (2013) 8:e58791. doi: 10.1371/journal.pone.0058791
138. Avila MA, Berasain C. Targeting CCL2/CCR2 in tumor-infiltrating macrophages: a tool emerging out of the box against hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. (2019) 7:293–4. doi: 10.1016/j.jcmgh.2018.11.002
139. Yang Z, Li H, Wang W, Zhang J, Jia S, Wang J, et al. CCL2/CCR2 axis promotes the progression of salivary adenoid cystic carcinoma via recruiting and reprogramming the tumor-associated macrophages. Front Oncol. (2019) 9:231. doi: 10.3389/fonc.2019.00231
140. Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. (1997) 385:640–4. doi: 10.1038/385640a0
141. Wojdasiewicz P, Poniatowski LA, Kotela A, Deszczynski J, Kotela I, Szukiewicz D. The chemokine CX3CL1 (fractalkine) and its receptor CX3CR1: occurrence and potential role in osteoarthritis. Arch Immunol Ther Exp. (2014) 62:395–403. doi: 10.1007/s00005-014-0275-0
142. Zhou B, Xu H, Ni K, Ni X, Shen J. Expression of chemokine XCL2 and CX3CL1 in lung cancer. Med Sci Monit. (2016) 22:1560–5. doi: 10.12659/MSM.895985
143. Wang Y, Fu Y, Xue S, Ai A, Chen H, Lyu Q, et al. The M2 polarization of macrophage induced by fractalkine in the endometriotic milieu enhances invasiveness of endometrial stromal cells. Int J Clin Exp Pathol. (2014) 7:194–203.
144. Geismann C, Erhart W, Grohmann F, Schreiber S, Schneider G, Schafer H, et al. TRAIL/NF-κB/CX3CL1 mediated onco-immuno crosstalk leading to TRAIL resistance of pancreatic cancer cell lines. Int J Mol Sci. (2018) 19:E1661. doi: 10.3390/ijms19061661
145. Reed JR, Stone MD, Beadnell TC, Ryu Y, Griffin TJ, Schwertfeger KL. Fibroblast growth factor receptor 1 activation in mammary tumor cells promotes macrophage recruitment in a CX3CL1-dependent manner. PLoS ONE. (2012) 7:e45877. doi: 10.1371/journal.pone.0045877
146. Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. (2009) 86:529–43. doi: 10.1189/jlb.0208125
147. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. (2004) 4:71–8. doi: 10.1038/nrc1256
148. Zhu YM, Webster SJ, Flower D, Woll PJ. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer. (2004) 91:1970–6. doi: 10.1038/sj.bjc.6602227
149. Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, et al. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. (2005) 23:953–64. doi: 10.1200/JCO.2005.12.172
150. Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. (2008) 14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843
151. Hosono M, Koma YI, Takase N, Urakawa N, Higashino N, Suemune K, et al. CXCL8 derived from tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression by promoting migration and invasion of cancer cells. Oncotarget. (2017) 8:106071–88. doi: 10.18632/oncotarget.22526
152. Sanmamed MF, Carranza-Rua O, Alfaro C, Onate C, Martin-Algarra S, Perez G, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. (2014) 20:5697–707. doi: 10.1158/1078-0432.CCR-13-3203
153. Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. (2017) 28:1988–95. doi: 10.1093/annonc/mdx190
154. Cury SS, de Moraes D, Freire PP, de Oliveira G, Marques DVP, Fernandez GJ, et al. Tumor transcriptome reveals high expression of IL-8 in non-small cell lung cancer patients with low pectoralis muscle area and reduced survival. Cancers. (2019) 11:E1251. doi: 10.3390/cancers11091251
155. Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, et al. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. (2003) 9:729–37.
156. Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F, et al. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int J Oncol. (2016) 48:5–12. doi: 10.3892/ijo.2015.3234
157. David JM, Dominguez C, Hamilton DH, Palena C. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines. (2016) 4:E22. doi: 10.3390/vaccines4030022
Keywords: lung cancer, tumor microenvironment, tumor-associated macrophages, cytokines, chemokines
Citation: Sarode P, Schaefer MB, Grimminger F, Seeger W and Savai R (2020) Macrophage and Tumor Cell Cross-Talk Is Fundamental for Lung Tumor Progression: We Need to Talk. Front. Oncol. 10:324. doi: 10.3389/fonc.2020.00324
Received: 30 September 2019; Accepted: 24 February 2020;
Published: 11 March 2020.
Edited by:
Edwin Ostrin, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Alexandre Corthay, Oslo University Hospital, NorwayBozena Kaminska, Nencki Institute of Experimental Biology (PAS), Poland
Copyright © 2020 Sarode, Schaefer, Grimminger, Seeger and Savai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajkumar Savai, rajkumar.savai@mpi-bn.mpg.de
 Poonam Sarode
Poonam Sarode Martina Barbara Schaefer2
Martina Barbara Schaefer2 Werner Seeger
Werner Seeger Rajkumar Savai
Rajkumar Savai