Article Text
Abstract
Background Conventional treatment of eosinophilic granulomatosis with polyangiitis (EGPA) (Churg–Strauss) with glucocorticoids, with or without additional immunosuppressive drugs, is limited by partial efficacy, frequent toxicity and a high relapse rate. Rituximab is a licensed treatment for granulomatosis with polyangiitis and microscopic polyangiitis and is of potential benefit to patients with EGPA.
Methods Patients with EGPA who received rituximab as single or repeated courses were identified from four vasculitis centres. Standardised data collection was performed, including disease activity status and adverse events, at the time of initial treatment and after 6 and 12 months. Remission was defined as a Birmingham Vasculitis Activity Score (BVAS) of 0 and partial response as a ≥50% reduction in BVAS compared with baseline.
Results 41 patients (21 women) with EGPA treated with rituximab between 2003 and 2013 were identified. 15 (37%) had refractory, 21 (51%) relapsing and 5 (12%) new onset disease. 19 received a single course and 22 received repeat-dose rituximab to prevent relapse. By 6 months, 83% improved with remission in 34% and partial response in 49%, and by 12 months 49% were in remission and 39% had a partial response. Prednisolone doses decreased in all patients by 6 and 12 months. Antineutrophil cytoplasmic antibody positivity at baseline was associated with a higher remission rate at 12 months. Adverse events included 15 infections (6 were severe).
Conclusions The treatment of EGPA with rituximab resulted in high rates of improvement and reduced requirement of prednisolone. Rituximab may be considered for the treatment of EGPA.
- Treatment
- Systemic vasculitis
- B cells
Statistics from Altmetric.com
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly named Churg–Strauss syndrome, is a rare multisystem small vessel vasculitis. EGPA is one of the antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides with myeloperoxidase (MPO) ANCA reported in 40% of cases.1 ,2 The disease has distinct clinical features, namely the presence of asthma, blood hypereosinophilia, tissue infiltration with eosinophils and necrotising granulomatous inflammation and vasculitis.1 ,3 In addition, ear–nose–throat involvement is a common feature of EGPA, reported in 48–77% of cases,2 ,4 and its presence is associated with good prognosis.5 Incidence rates of 2.7/million in the UK6 and 2.3/million in Australia have been reported.7 The prevalence of EGPA is between 11 and 24/million in Europe.8–10 The prognosis of EGPA varies depending on organ involvement. European League Against Rheumatism recommendations suggest a combination of glucocorticoids and pulsed intravenous cyclophosphamide (CYC) infusions for the treatment of generalised primary small and medium vessel vasculitis including EGPA.11 Patients with severe disease and poor prognostic features may require longer CYC duration to control disease activity and decrease relapse rates.12 However, even in patients without poor prognostic features, treatment with glucocorticoids alone is not optimally effective due to the high-dose requirement and high relapse rate.13
Rituximab is an anti-CD20 monoclonal antibody directed against B cells, which are believed to play a central role in pathogenesis of ANCA-associated vasculitis (AAV). Following two randomised controlled clinical trials demonstrating the efficacy of rituximab as induction treatment, rituximab is licensed for the treatment of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) both in Europe and the USA.14 ,15 Patients with EGPA were excluded from these studies, and there has not been a randomised controlled clinical trial conducted to study the use of rituximab in EGPA. However, data from a small number of case reports and small case series have indicated rituximab may be effective in patients with refractory or relapsing EGPA.16–24 Interleukin 5 (IL-5) plays a central role in stimulation and maturation of eosinophils, which contribute to the pathogenic processes in EGPA. The B-cell-dependent activation of T-lymphocytes with subsequent IL-5 production is a possible mechanism of action of rituximab in EGPA.25
The aim of this study is to report the experience of using rituximab in EGPA at four specialised vasculitis centres in Europe and the USA including the response to treatment with rituximab and adverse events within the first year of initial infusion.
Patients and methods
Forty-one patients (21 women) with a diagnosis of EGPA who were treated with rituximab between 2003 and 2013 at four centres were included in this retrospective study: Vasculitis Center, Boston University Medical School, Boston, Massachusetts, USA (n=4); Department of Internal Medicine, Hôpital Cochin, Paris, France (n=2); Rheumatologic Clinic, Bad Bramstedt, Germany (n=9); and the Vasculitis and Lupus Clinic at Addenbrooke's Hospital, Cambridge, UK (n=26). All patients fulfilled the American College of Rheumatology 1990 classification criteria for Churg–Strauss syndrome.26 The majority (36/41, 88%) had either refractory disease (15/41, 37%) or relapsing disease (21/41, 51%). The remaining five received rituximab as first-line therapy, for CYC avoidance, due to concerns regarding infertility or due to significant comorbidities.
Treatment protocols
In total, 19/41 patients received a single course of rituximab. Re-treatment was given for 22/41 at 6 months and 17/22 were re-treated again at 12 months. Two received their first re-treatment at 12 months. The initial treatment schedule was 375 mg/m2/week for 4 weeks (n=10) or two doses of 1000 mg given 2 weeks apart (n=30). One patient received two doses of 800 mg at a 2-week interval. Subsequent rituximab courses and doses were 375 mg/m2/week for 4 weeks (3 patients), two doses of 1000 mg 2 weeks apart (2 patients), 1000 mg single dose (16 patients), and a single dose of 600 mg rituximab (1 patient). The majority of patients received premedication with intravenous hydrocortisone 100 mg, intravenous chlorpheniramine 10 mg or diphenhydramine 50 mg, and oral acetaminophen 1 g before each rituximab infusion.
Data collection
Data collection included baseline demographics, laboratory parameters and disease activity assessments at entry (time of first rituximab treatment) and sequentially at 3, 6, 9 and 12 months after initial rituximab infusion. Organ involvement at any point during the disease course was assessed according to the Disease Extent Index (DEI).27 In DEI, asthma was considered as a surrogate for lung involvement. Data about previous immunosuppressive drugs as well as prednisolone/prednisone doses at entry were collected. A positive ANCA result was recorded if either immunofluorescence or ELISA for either proteinase-3 (PR3-) or MPO-ANCA were positive. CD19+ B-cell depletion was defined as a level of 0.00×109/L. B-cell reconstitution defined as a level of ≥0.01×109/L. Disease activity was assessed using the Birmingham Vasculitis Activity Score (BVASv3).28 In BVAS, symptomatic asthma was scored as ‘wheeze’.
Treatment outcome and adverse events
The treating physician judged the response to therapy clinically. Remission was defined as a BVAS of zero. Partial response was defined as reduction of ≥50% in the BVAS compared with baseline score. Relapse was defined as the recurrence of signs and symptoms of EGPA, which led to reinstitution of any immunosuppressive therapy or an increased glucocorticoid requirement and an increase in BVAS. Refractory disease was defined as failure to attain remission after conventional immunosuppressive treatments, including biologics, and high-dose steroids. Infusion reactions were defined as any allergic skin reactions, hypotension, difficulty in breathing or unexplained circulatory deterioration that developed during or within few hours of rituximab administration. Mild hypogammaglobulinemia was defined as an IgG level between 5 and 6.9 g/L; moderate between 3 and 4.9 g/L and severe if IgG level <3 g/L. Severe adverse events were those resulting in hospitalisation, prolongation of an existing hospital stay, the use of intravenous antibiotics treatment for infections or the diagnosis of cancer.
Statistical analyses
Differences in the frequency of organ involvement or other categorical variables between groups were studied using Fisher's exact test and χ2 test. Continuous variables were presented as medians and IQRs unless otherwise stated. Differences in continuous variables were tested by the non-parametric Student t test or Mann–Whitney U test. Differences in continuous variables within groups were analysed using the paired sample t test. A p value of <0.05 was considered significant for all analyses. Statistical analyses were performed using the Statistical Package for the Social Sciences; SPSS V.22.0 for Windows (IBM SPSS V.22.0.0).
Results
Forty-one patients (21 women) were included in this study. The demographic and clinical characteristics of patients are shown in table 1.
Baseline demographics and clinical characteristics of 41 patients with eosinophilic granulomatosis with polyangiitis treated with rituximab
Response to treatment
The median BVAS at baseline was 11 (IQR 6–17.5) and decreased to 2 (0–6.2) at 6 months (p<0.001) and to 1 (0–2) at 12 months (p<0.001, vs baseline and p=0.017 vs 6 months) (figure 1). Treatment with rituximab was associated with improvement in 34/41 (83%) patients by 6 months and 36/41 (88%) by 12 months. The proportions of patients who achieved remission by 6 and 12 months were 14/41 (34%) and 20/41 (49%), respectively. The corresponding rates for partial response by 6 and 12 months were 20/41 (49%) and 16/41 (39%), respectively. Partial response or remission was achieved at 3 months in 31/36 patients (86%), mainly partial response (64%). There was a steady increase in the proportion of patients achieving remission over the year following rituximab (figure 2). Patients with positive ANCA testing were more likely to achieve remission: 12/15 (80%) patients who were ANCA-positive achieved remission at 12 months compared with 8/21 (38%) who were ANCA-negative (p=0.013). There was no difference in the proportion of remission and partial response among patients who received rituximab for refractory or relapsing disease. All patients with renal involvement who improved (7/10) achieved remission (p=0.008). No other clinical or demographic factors were predictive for not achieving remission (see online supplementary table S1). Each of the five who received rituximab as a first agent improved with three in remission at 12 months (see online supplementary figure S1 details for the characteristics and response rate of refractory and relapsing cases and patients received rituximab as first line).
Median (IQR) of the Birmingham Vasculitis Activity Score (BVAS) at baseline, and at 3, 6, 9 and 12 months after initial rituximab treatment. Baseline: 11.0 (6.0–17.5) vs 6 months: 2.0 (0.0–6.2), p<0.001 and 12 months: 1.0 (0.0–2.0), p<0.001.
Percentage of patients who were in remission, partial response (PR) and no response (NR) at 3, 6, 9 and 12 months after first rituximab treatment.
At 6 months, seven patients (17%) had not improved. Six of these seven patients (three were ANCA-positive) were slow responders and achieved remission (3) or partial response (3) by 12 months, four were re-treated at 6 months and two received only single course of rituximab at entry. One patient was a true non-responder showing no signs of response throughout the 12 months following rituximab (despite re-treatment at 6 months). Additionally, 4/34 patients (12%) who had improved (two remission, two response) at 6 months experienced relapse at 12 months. Among the 20 patients who were in remission at 12 months, 13 (65%) were treated with a single course of rituximab.
Type and rate of response did not differ between the patients initially treated with rituximab 375 mg/m2/week for 4 weeks (n=10, group 1) or patients treated with rituximab two doses of 1 g given 2 weeks apart (n=30, group 2). All group 1 patients improved (8 with remission) at 12 months compared with 25/30 (83%), 11 with remission, of group 2, p=0.053.
Prednisolone
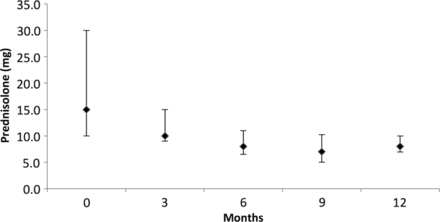
The median prednisolone/prednisone dose was 15 mg/day at baseline (IQR 10–30 mg) and decreased to 8 mg/day (IQR 6.5–11) at 6 months (p<0.001) and 8 mg/day (IQR 6.9–10) at 12 months (p=0.001, vs entry dose) (figure 3). The results were unchanged when the analyses were repeated after stratifying patients into two groups: those receiving single versus repeated courses of rituximab. Among patients who received a single course of rituximab (n=19), the median prednisolone dose at entry was 20 mg/day (IQR 10–40), 8 mg/day (IQR 5.5–10.7) at 6 months (p=0.006) and 7 mg/day (IQR 6–13) at 12 months (p=0.024, vs entry dose). The corresponding figures among patients treated with repeated courses (n=22) were 15 mg/day (IQR 10–30) at entry, 8 mg/day (IQR 6.5–11.5) at 6 months (p=0.013) and 8 mg/day (IQR 6.8–10) at 12 months (p=0.027, vs entry dose).
Median (IQR) prednisolone/prednisone dose at baseline, and 3, 6, 9 and 12 months after initial rituximab treatment. Baseline: 15 (10–30) vs 6 months: 8 (6.5–11), p<0.001 and 12 months: 8 (6.9–10), p=0.001.
At entry, among the 39 patients who were on prednisolone, only 2 (5%) were on a daily dose <10 mg. The number of patients on <10 mg/day of prednisolone increased to 16/31 (52%) at 6 months (p<0.001) and 17/32 (53%) at 12 months after initial rituximab treatment (p<0.001 vs entry). At 6 and 12 months after initial treatment, only two (6%) were on no prednisolone.
For the five patients who received rituximab as first induction therapy, the median prednisolone dose at entry was 25 mg (IQR 17.5–70) and decreased to 6 mg at 12 months (IQR 1.25–9.25).
Other immunosuppressive drugs
In total, 36/41 patients (88%) received at least one immunosuppressive drug other than prednisolone prior to rituximab (table 1). At entry 16/36 (44%) patients were on an immunosuppressive drug (mycophenolate=7, azathioprine and CYC=3 each, methotrexate=2 and intravenous immunoglobulin=1); the number decreased at 12 months to 10/36 (28%) (azathioprine=5 and methotrexate=5), p=0.140.
Laboratory findings
B-cell depletion occurred in all patients. At entry, the median B-cell count for all patients was 0.08 ×109/L (IQR 0.04–0.25), depleted to zero at 3 months, and remain so throughout the 12 months (median 0.00, IQR 0.00–0.00) (p=0.030). At 12 months, data on B-cell counts were available for 17 of which only 2 (12%) had reconstituted B cells. The inflammatory parameters and creatinine were unchanged through the study period (table 2). There was no difference in eosinophil counts at 6 and 12 months (p=0.054).
Laboratory data at entry (time of first rituximab infusion) and at 12 months
Data on IgG levels were available for 35 patients at baseline, and for 27 and 22 patients at 6 and 12 months, respectively. The median IgG level at baseline was 8.4 (7.4–10.6) g/L, and decreased at 12 months, to 7.6 g/L (6.2–8.7), p=0.044, but was still within normal range (table 2). At baseline, 6/35 patients (17%) had mild hypogammaglobulinemia, which increased to 9/27 (33%) at 6 months (p=0.139) and to 8/22 (36%) at 12 months (p=0.100). There were no differences in IgG levels between groups that had received single or multiple rituximab courses (data not shown).
Adverse events
Thirty-one adverse events were recorded in 21/41 (51%) patients who received a total of 79 rituximab courses (table 3). Also, 12/21 had received a single rituximab course and the remaining nine had been treated with repeated courses (p=0.075). The most common adverse events were infections, followed by allergic reactions. In total, 15 infections occurred in 14 patients. Among infectious adverse events, six were severe: chest infections (n=3), upper respiratory tract infections (n=2) and pyelonephritis (n=1). Ten infusion reactions occurred in seven (17%) patients. Eight of ten allergic reactions were mild and abated after temporarily stopping the rituximab infusions and adding glucocorticoids. In the other two cases, the infusion reactions were severe and required admission to hospital and treatment with intravenous glucocorticoids (of which one required intubation due to worsening of asthma). There were no cases of rituximab-related late-onset leucopoenia. Other serious adverse events included acute confusion state (n=1), and in one patient (a 40-year-old woman) a thymoma was diagnosed at 3 months after first rituximab treatment. No deaths occurred within 1 year of the first rituximab treatment.
Adverse events within 1 year of first rituximab treatment in patients with eosinophilic granulomatosis with polyangiitis
There were no differences in disease duration prior to rituximab, age at first infusion, BVAS, B-cell counts, IgG levels or prednisolone dose between patients who developed any adverse events or infections and those who did not (data not shown).
Discussion
In this study, we present data on 41 patients with EGPA treated with rituximab in four vasculitis centres. Improvements in disease activity (remission and partial response) were achieved in 83% of patients at 6 months after the first infusion with rituximab. At 12 months, the improvement rate was nearly 90%. Despite the refractory and relapsing nature of EGPA in many patients in this study, this efficacy rate was high with an acceptable adverse event profile.
Despite the reduction of prednisolone dose at 6 and 12 months compared with baseline doses, only 6% of patients were off all treatment with prednisolone by the end of the observation period, indicating that rituximab in this cohort was not a fully glucocorticoid-sparing agent. This is in contrast to patients with GPA and MPA who received scheduled rituximab treatment for relapsing disease in whom prednisolone treatment was successfully withdrawn by 24 months.29 The inability to withdraw prednisolone in EGPA has been reported in previous studies using conventional cytotoxic therapies. In a study from the French Vasculitis Study group,13 52/66 patients who were in remission had a mean prednisolone dose of 9 mg/day at 60 months of follow-up with the prednisolone prescribed primarily for control of asthma.
We found no difference in the rate of response, type of response or number of adverse events between patients with relapsing or refractory disease. There was no difference in the rate of response between patients who received rituximab 375 mg/m2/week for 4 weeks or those who received rituximab two doses of 1 g at 2-week intervals.
Rituximab led to improvements among the majority of patients with EGPA who were ANCA-negative in this study but appeared more effective in those ANCA-positive, as judged by remission rates. Among reported cases of EGPA treated with rituximab, 15/21 (71%) were ANCA-positive and 6/21 (29%) were ANCA-negative.16–24 Although the randomised trials of rituximab for GPA/MPA were limited to ANCA-positive patients,14 ,15 cohort studies have reported similar high response rates in ANCA-negative GPA/MPA.30 ,31 Thus, the absence of ANCA should not be a barrier to use of rituximab to treat EGPA.
The inflammatory markers in this study were unchanged during the 12 months after treatment with rituximab even when analyses were limited to patients who achieved remission (data not shown). There was no difference in the total eosinophil count at 12 months compared with entry. The majority of cases in this study have been on variety of prior immunosuppression therapies, which might result in modulation of immune response and limited or minimised any changes in laboratory markers to assess disease activity. These findings highlight the need for other biological indices to assess disease activity.
IgG levels decreased at 12 months compared with baseline but remained within normal range. No patient developed severe hypogammaglobulinemia. The proportion of patients developing mild hypogammaglobulinemia in this study was comparable to previous experience using rituximab as maintenance therapy in ANCA-associated vasculitis29 and EGPA.23 The mild hypogammaglobulinemia did not affect the outcome of therapy in terms of response rate or in frequency of adverse events.
Rituximab was well tolerated in our patients, and the frequency of severe adverse events was comparable with other experience with rituximab in AAV.29 However, we observed a relatively high rate of infusion reactions, although most were mild. Our findings support the use of premedication with intravenous glucocorticoids and antihistamines to minimise allergic reactions in patients with EGPA. These results are in accordance with the safety profile of rituximab in EGPA reported from 22 cases.16–24 Prophylactic antibiotic treatment was not routinely prescribed, but no opportunistic infections occurred.
Our study has several limitations. First, the retrospective nature was associated with some missing data and difficulty in assessing disease activity. Second, being a multicentre study different treatment protocols were followed when using other immunosuppressive drugs in combination with rituximab. Furthermore, we cannot exclude a selection bias as all cases are from highly specialised vasculitis centres. Nevertheless, the strength of this study is in being the largest study on the use of rituximab in patients with EGPA and presents the experiences of specialised centres in care of patients with EGPA in different countries.
In conclusion, we have shown that rituximab is a safe and important alternative to standard therapy in EGPA, both for refractory and relapsing diseases, especially for ANCA-positive patients, and also for newly diagnosed patients in whom traditional cytotoxic drugs are contraindicated or undesirable. Despite the good response rate in this study, complete withdrawal of glucocorticoids was generally not feasible, highlighting the unmet need for glucocorticoid-sparing agents in EGPA. Long-term outcome of the use of rituximab in EGPA and its role as maintenance treatment need to be addressed in future studies.
Acknowledgments
We are grateful to Dr Vega Martinez for assistance with data collection. This project was supported by the Cambridge Biomedical Research Centre and The Swedish Society of Medicine (Svenska Läkarsällskapet).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
Handling editor Tore K Kvien
Contributors Study design: AJM, PAM and DRWJ. Data collection: AJM, AH, NA, FA and LG. All authors were involved in drafting the article or revising it critically and approved the final version. AJM had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analysis: AJM.
Funding Cambridge Biomedical Research Centre and The Swedish Society of Medicine (Svenska Läkarsällskapet).
Competing interests None.
Ethics approval Obtained from individual institutional ethics boards.
Provenance and peer review Not commissioned; externally peer reviewed.