Article Text
Abstract
The use of continuous positive airway pressure (CPAP) in treating symptoms associated with OSAHS is reviewed. Although it is an imperfect intervention, it continues to evolve and improve in such a way that patients who would not have been able to use this treatment even in the recent past can benefit from it today.
- AHI, apnoea hypopnoea index
- CHF, congestive heart failure
- CPAP, continuous positive airway pressure
- CSB, Cheyne-Stokes breathing
- OSAHS, obstructive sleep apnoea/hypopnoea syndrome
- PAP, positive airway pressure
- obstructive sleep apnoea/hypopnoea syndrome
- continuous positive airway pressure
Statistics from Altmetric.com
- AHI, apnoea hypopnoea index
- CHF, congestive heart failure
- CPAP, continuous positive airway pressure
- CSB, Cheyne-Stokes breathing
- OSAHS, obstructive sleep apnoea/hypopnoea syndrome
- PAP, positive airway pressure
Obstructive sleep apnoea/hypopnoea (OSAHS) is a common disorder which is characterised by instability of the upper airway during sleep resulting in reduction or elimination of airflow, oxyhaemoglobin desaturation, and sleep disruption. The prevalence of OSAHS has been estimated at 24% in men and 9% in women.1 There is increasing evidence to support OSAHS as a risk factor for cardiovascular and cerebrovascular disorders including hypertension, congestive heart failure, myocardial infarction, and stroke.2–5 In addition, OSAHS is associated with impaired neurocognitive function and alertness believed to contribute to the increased rates of motor vehicle crashes and traffic fatalities.6–9 Thus, in view of the considerable exposure within the general population to OSAHS and its notable consequences, expeditious diagnosis and effective treatment of these patients has important individual as well as public health benefits.
The mainstay of medical treatment of OSAHS is administration of non-invasive positive airway pressure (PAP) during sleep. In this review we will summarise the available data on PAP treatment for OSAHS, focusing on current hypotheses regarding possible mechanisms of action, evidence for its impact on OSAHS associated morbidities, and data describing populations which may or may not benefit from PAP.
MECHANISM(S) BY WHICH PAP STABILISES THE UPPER AIRWAY DURING SLEEP
Although continuous positive airway pressure (CPAP) has been used to treat patients with OSAHS for over two decades, our understanding regarding the mechanism of action continues to evolve. The most widely accepted view is that the positive pressure provides a mechanical stent of the upper airway (fig 1). This theory was first suggested by Sullivan et al in 198110 and was subsequently supported by Alex et al11 who compared the relationship between oesophageal pressure swings and inspiratory airflow during breathing without and with CPAP in normal awake subjects. These investigators observed that, at a given oesophageal pressure, inspiratory airflow was greater during CPAP administration despite the presence of decreased genioglossus muscle activation compared with baseline.
Mechanism by which positive pressure stents open the upper airway. Note the closure of the oropharynx by the soft palate, reducing air leak through the mouth. Reprinted with permission from the slide set of the American Academy of Sleep Medicine.
Some investigators have suggested that increases in lung volume during PAP administration mediate the upper airway stabilising effect of this treatment. This concept is based on data indicating that patients with OSAHS have greater lung volume dependency of pharyngeal cross sectional area than individuals without OSAHS.12,13 In other words, pharyngeal cross sectional area, measured during wakefulness by the technique of acoustic reflection, decreases to a greater extent in patients with OSAHS than in normal individuals as lung volume decreases.12,14 Two mechanistic theories have been proposed to explain this modulation. One suggests that PAP associated augmentation of lung volume elicits a reflex which increases upper airway dilator muscle tone. The other theory proposes that forces associated with increased lung volume are transmitted to the upper airway via the trachea (fig 2). The resulting stretch or “tracheal tug” stiffens and stabilises the upper airway.15,16 The feasibility of tracheal tug as a mediator of upper airway resistance has been supported by experiments using animal models.17–20
Possible mechanisms by which “tracheal tug” influences upper airway patency. Reprinted with permission from Van de Graaff.17
Regardless of the proposed mechanisms through which changes in lung volume may modulate upper airway stability, the evidence regarding the operational significance of this pathway is, at best, contradictory. Support for reflex augmentation of upper airway dilator muscle activity is diminished in separate reports by Strohl et al21 and Rapoport et al22 who indicated that genioglossus muscle EMG activity is either reduced or variable during CPAP use which eliminates obstructive events.
Although Sériès et al suggested that augmentation of end expiratory lung volume by application of negative end expiratory pressure using an “iron lung” reduced the frequency of apnoea during sleep,23 other investigators using the same technique failed to observe a favourable effect.24 In addition, a separate study by Sériès et al14 showed that upper airway resistance progressively decreases during administration of increasing levels of CPAP even when lung volume is maintained by application of positive extrathoracic pressure. Thus, it appears that the primary mechanism for improvement in upper airway stabilisation by PAP administration is related to a mechanical splinting effect.
OUTCOMES OF TREATMENT WITH PAP
Optimising patient acceptance and adherence to PAP treatment remains a major clinical challenge. Significant work has been done to determine which patients with OSAHS will adhere to a PAP prescription and who will derive physiological and quality of life benefits from this treatment. It has been recommended that all symptomatic patients with OSAHS (who by definition have an apnoea hypopnoea index, AHI, of >5) should receive treatment.25 The Amercian Academy of Sleep Medicine (formerly the American Sleep Disorders Association) guidelines for CPAP therapy recommend its use for patients with an apnoea index (mean number of apnoeas per hour of sleep) of ⩾20 and for symptomatic patients with an AHI (mean number of apneas+hypopneas per hour of sleep) or respiratory arousal index (mean number of arousals per hour of sleep) of ⩾10.26 In the United States Medicare will cover CPAP devices for patients with an AHI of ⩾15, or patients with sequelae of OSAHS and an AHI of 5–14. Such sequelae include excessive daytime sleepiness, impaired cognition, hypertension, coronary artery disease, cerebrovascular accident, mood disorders, and insomnia.27
Sleepiness and alertness
When compared with sham CPAP, it has not been proved that asymptomatic patients with severe OSAHS have improved daytime function or decreased sleepiness after 6 weeks of CPAP.28 On the other hand, symptomatic patients with mild OSAHS (AHI 5–15) as well as severe OSAHS have been shown to experience a reduction in subjective as well as objective measures of daytime sleepiness with CPAP treatment.29–36 Several studies, including those employing subtherapeutic levels of CPAP as a control, have demonstrated observed improved quality of life in symptomatic patients with even mild OSA HS.37–40
Additional support for the salutary impact of CPAP on daytime sleepiness, as well as demonstrating the importance of patient adherence to treatment, was provided by Kribbs et al41 who reported that daytime sleepiness (measured by the Epworth Sleepiness Scale and Maintenance of Wakefulness Test) improved significantly after prolonged therapeutic CPAP. However, just one night without treatment was sufficient to reverse these improvements, despite a reduced AHI compared with pretreatment levels (mean AHI of 37 during one night off CPAP following chronic treatment v pretreatment AHI of 56).
Neurocognition and performance
OSAHS adversely affects certain neurocognitive functions including memory and learning, as well as task performance such as driving.42–44 Studies have found improved cognitive performance after adequate CPAP treatment. Montserrat et al reported that, compared with placebo, CPAP was associated with significantly improved daytime function as measured by the Functional Outcomes Sleep Questionnaire (FOSQ).40 Engleman observed that even an average CPAP use of 3.4 hours per night was associated with improved cognitive task performance and attention.30
Motor vehicle crashes represent another major morbidity and mortality attributable to OSAHS.6–9 Engleman and coworkers noted that patients with higher levels of adherence to treatment with CPAP reported fewer traffic accidents relative to the pretreatment period.45 Additional studies have confirmed the beneficial effects of therapeutic CPAP on driving performance and motor vehicle crashes.46,47
In summary, CPAP use has clearly been shown to have a favourable impact on daytime sleepiness in sleepy patients with OSAHS, driving performance as measured by driving simulators, and also accident rates. In addition, even 3–4 hours per night of CPAP use can improve measures of cognition and daytime vigilance.
Cardiovascular outcomes
There is growing recognition that OSAHS is associated with a notably increased risk for adverse cardiovascular and cerebrovascular consequences.2,4,5,48 The risk for hypertension engendered by OSAHS is increasingly acknowledged2,48–52 but the impact of PAP on hypertension remains controversial. Dimsdale et al reported a decrease in nocturnal blood pressure but no decrease in diurnal hypertension with CPAP use in patients with OSAHS and hypertension.53 However, several other studies have found that CPAP therapy reduces diurnal and nocturnal blood pressure in hypertensive patients with OSAHS.54–58 Although small, this reduction in blood pressure may have meaningful health benefits.59
Congestive heart failure (CHF) may be associated with sleep disordered breathing, including OSAH, central sleep apnoea, and Cheyne-Stokes breathing (CSB).60 Left ventricular failure, which can be worsened by the exaggerated intrathoracic pressure swings during apnoeas and hypopnoeas,61 is improved by use of nasal CPAP.62–69 Furthermore, recent data suggest that CPAP administration to patients with CHF reduces morbidity with improved New York Heart Association functional class and has a favourable impact on mortality in patients with CSB.67 Malone et al reported improved left ventricular ejection fraction (LVEF) in CHF patients with OSAHS after CPAP and a reduction in LVEF during temporary withdrawal of CPAP.61 Krieger et al reported that CPAP normalised LVEF as well atrial natriuretic peptide (ANP) in patients with mild CHF and sleep apnoea.70,71 CSB is an independent predictor of mortality from CHF. CPAP has been shown to eliminate CSB and decrease mortality in patients with this pattern of periodic breathing.67 There is some question as to whether CPAP confers any added benefit in reducing CHF morbidity beyond oxygen therapy alone, although this question continues to be an area of active investigation.72
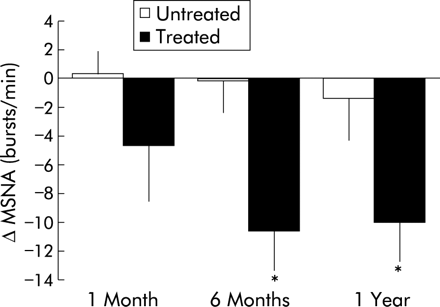
The mechanism by which CPAP improves cardiovascular outcomes is unknown, although there is increasing evidence that a reduction in sympathetic tone may play an important role. Adverse cardiac consequences in patients with OSAHS may be attributable to the increaseded sympathetic autonomic activity that has been noted in these patients during wakefulness as well as sleep.73–76 Hypoxia, and potentially hypercapnia, resulting from apnoeas and hypopnoeas may account for this increased sympathetic activity during sleep, although the mechanism for increased diurnal autonomic activity is less clear. Importantly, prolonged CPAP therapy significantly reduces diurnal sympathetic tone in patients with OSAHS (fig 3).68,74,77,78 It has been speculated that the reduction in sympathetic activity in patients with OSAHS associated with CPAP use may have a favourable effect on cardiovascular outcomes in much the same way as beta blockade, although this has not been shown to date. Further investigation is warranted to clarify these findings.
Change in muscle sympathetic nerve activity (MSNA) in untreated patients with sleep apnoea (n = 9) and in patients treated with CPAP at 1 month, 6 months, and 1 year (n = 11). Sympathetic activity decreased significantly in the treated patients but not in the untreated patients. Reprinted with permission from Narkiewicz et al.77
Dean et al79 found evidence of increased atherogenesis in OSAHS. Since this study there has been significant work regarding the relationship between OSAHS and vascular endothelial cell function, as well as the potential modifying role of CPAP therapy in this regard.80,81 OSAHS has been associated with increased levels of soluble cell adhesion molecules which are correlated with atherosclerosis. It may therefore be significant that CPAP therapy reduces levels of E-selectin and soluble intercellular adhesion molecule-1, with persistence of this effect through 6 months of treatment.80
Duchna et al81 reported evidence of impaired endothelial cell function in patients with OSAHS with impaired vasodilation in response to bradykinin and nitroglycerin. This dysfunction could contribute to the increased cardiovascular and cerebrovascular morbidity associated with OSAHS. It is noteworthy therefore that the vasodilatory responsiveness to bradykinin increased significantly and there was a trend towards increased vasodilation in response to nitroglycerin following 60 days of CPAP treatment.
Leptin levels are raised in patients with OSAHS.82 This may be significant in that leptin modulates the degree and distribution of fat deposition with increased visceral fat having an association with coronary heart disease. Furthermore, leptin may be associated with increased sympathetic activation83,84 with a consequent adverse cardiovascular impact. In this context, it may be noteworthy that CPAP has been shown to reduce serum leptin levels and decrease the proportion of visceral fat in OSAHS patients even when overall weight is unchanged.85
Taken together, the evidence listed above provides strong evidence that CPAP favourably modifies multiple factors at the neurophysiological and molecular levels which promote cardiovascular disease.
ACCEPTANCE OF AND ADHERENCE TO CPAP
Acceptance refers to the patient’s perception of CPAP and willingness to consider its use. Acceptance is a necessary first step to adherence but should be considered separately. Estimated rates of acceptance range from 72% to 91%.86–91 Estimates of adherence to CPAP therapy vary, but overall adherence is suboptimal. Kribbs and colleagues found that when adherence was defined as use for at least 4 hours on at least 70% of nights, only 46% of patients qualified.92 Subsequently, Engleman et al reported a mean daily CPAP use of 4.7 hours.93 Not only may the mean number of hours of CPAP use vary across patients, but so may the pattern of use. Weaver et al reported that adherence followed a bimodal distribution in which half of patients used CPAP at least 90% of nights with a mean of 6.22 hours of nightly use while the other half used CPAP intermittently and nightly use varied greatly.94 Attempts have been made to identify factors associated with higher rates of use, as the degree of adherence positively correlates with patient outcomes.38,45 In evaluating 1200 patients with OSAHS, McArdle and coworkers found that independent predictors of self-reported 5 year adherence to CPAP included presence of snoring, magnitude of AHI, and degree of sleepiness.95 Engleman found that, in patients with mild OSAHS, those who were more adherent to treatment had higher AHIs than those who used CPAP less regularly.96 On the other hand, other studies have found no association between AHI and adherence to treatment.37,90,93,97,98 The incidence of adverse effects was inversely correlated with CPAP use in one study.45
From a clinical management perspective, it is important to note the observation that intermittent CPAP use within the first few weeks following initiation of treatment is associated with shorter nightly use,94 because it suggests that clinicians have a brief window of opportunity to address potential impediments to adherence before patients establish an unacceptable pattern of use. Suboptimal adherence to CPAP therapy notwithstanding, it is important for clinicians to appreciate that the rates of adherence are comparable with other treatments such as metered dose inhalers (MDI) in asthma for which adherence has been reported as 37–52% when objectively measured.99,100 Thus, although adherence to CPAP remains suboptimal, it is similar to or better than that of less intrusive treatments for other disorders.
Suboptimal adherence has been a motivator for the development of PAP modalities as alternatives to conventional CPAP which will be discussed later in this article. Other strategies have included intensifying follow up of patients with OSAHS on PAP therapy. In a prospective randomised trial, Hoy et al101 implemented a programme of intensive education and adaptation, including three nights of CPAP trials in the sleep facility and subsequent rigorous follow up. The patients randomised to intensive support were significantly more adherent to treatment at 1, 3, and 6 months than were the patients randomised to usual care (fig 4).101 In contrast, employing a similar study design, Hui et al102 found no difference in CPAP use between groups randomised to usual care or “augmented support”.
Nightly adherence to CPAP at 1, 3, and 6 months in sleep apnoea patients with intensive (filled bars) versus standard (shaded bars) support. The difference between groups is significant (p = 0.003). Reprinted with permission from Hoy et al.101
SIDE EFFECTS/ADVERSE EFFECTS
Engleman reported that the incidence of side effects directly affected patient adherence to treatment,93 although other studies have not found such a correlation.98,103
Regardless of the nature of the relationship between side effects and adherence, it seems prudent to minimise any adverse impacts associated with PAP therapy. In general, these can be categorised as those related to nasopharyngeal symptoms, those related to the interface or nasal route of delivery, and those specifically related to the magnitude of pressure.
The most often reported nasopharyngeal symptoms include increased congestion and rhinorrhoea. These are common and may be related to release of inflammatory mediators as a result of reduced relative humidity in the inspired gas.104 Humidification of the delivered gas may improve this symptom.104–108 Non-heated humidifiers have been used in this regard but refractory patients may benefit from incorporation of a heated humidifier into the PAP circuit.105–108 Recent data suggest that elderly patients, individuals who are on medications that may cause mucosal dryness, patients with chronic nasal symptoms, and those who have had a uvulopalatopharyngoplasty are most likely to require a heated humidifier.108 In selected patients use of an oronasal mask may increase the relative humidity of the inspired gas,109 although one study suggested that this type of interface is less well tolerated than nasal interfaces.110 Humidification may reduce the likelihood of epistaxis.
Clinicians may prescribe topical nasal steroids or ipratropium nasal spray to treat nasal complaints associated with PAP therapy. Although we have observed that some patients report improvement following the former treatment, we are unaware of systematic studies evaluating these interventions. Patients who experience oral dryness due to inability to keep the mouth closed during nasal CPAP therapy may derive benefit from a chinstrap or an oronasal mask.111
CPAP therapy for OSAHS was initially delivered exclusively via the nasal route. Over the years a number of nasal masks and nasal prong interfaces have evolved and continue to do so in an effort to enhance patient comfort. In addition, some patients have a preference for oronasal masks which do not require exclusively nasal breathing.111,112
Adverse consequences of PAP treatment are often related to poorly fitting masks and include skin breakdown and air leaks.113 Air leaks at the skin-mask interface or through the mouth when using a nasal interface may preclude achievement of the prescribed pressure and lead to inadequate treatment. If air leaks are directed towards the eyes, conjunctivitis may ensue. Local skin reactions to the interface have also been reported.113,114 Air leaks may also disrupt sleep. In general, interface-related problems can be resolved for most patients by careful and methodical assessment of all interface options.
Finally, the pressure applied to the upper airway and lungs can result in adverse consequences. Patients may report chest and ear discomfort as well as discomfort associated with exhaling against high pressures.89,98 Increased intraocular pressure has also been observed,115 as has barotrauma. Although uncommon, barotrauma has been reported in conjunction with PAP therapy, including pneumoencephalus116 and pneumothorax.117 Clinicians should remain aware of the potential risk of PAP in patients at high risk such as those with bullous lung disease.
MODALITIES OF PAP
By definition, CPAP reflects delivery of the same magnitude of pressure during both inspiration and expiration. In addition to conventional CPAP, there are other modalities for application of non-invasive PAP.
Bilevel positive airway pressure
Unlike CPAP which, by definition, provides the same magnitude of pressure during the inspiratory and expiratory portions of the ventilatory cycle, bilevel positive airway pressure (bilevel PAP) permits independent adjustment of the inspiratory and expiratory positive airway pressures (IPAP and EPAP, respectively). The rationale underlying application of this modality is derived from observations that upper airway closure during sleep in OSAHS is an expiratory as well as an inspiratory phase related event.118,119 The EPAP level is set to stabilise the upper airway at end expiration such that the airway is sufficiently patent to permit the patient to trigger delivery of IPAP by generating low level inspiratory volume or flow during the subsequent effort. The IPAP level is set to prevent upper airway closure and partial obstruction (hypopnea) during the inspiratory phase of breathing. Since it usually requires less pressure to keep the upper airway adequately patent during expiration than during inspiration, the requisite EPAP is usually lower than the IPAP.120 This differs from conventional CPAP in which the pressure delivered during expiration must be as high as that delivered during inspiration. In the “spontaneous mode” of bilevel PAP the patient may breathe with his/her own breathing pattern. Some currently available bilevel PAP devices incorporate the option for the clinician to prescribe a “backup rate” to guarantee a certain number of pressure cycles (or breaths) per minute.
The benefits of bilevel therapy fall largely into two categories: providing ventilatory assistance and improved patient comfort. Resta et al121 and Schafer et al122 independently found that patients with OSAHS which was refractory to CPAP may be successfully treated with bilevel PAP. Patients who failed on CPAP treatment (persistent apnoea or desaturation) and subsequently had successful alleviation of sleep disordered breathing tended to be more obese with a lower arterial oxygen tension (Pao2) and higher arterial carbon dioxide tension (Paco2) during wakefulness. Resta et al121 observed that OSAH patients with co-existent chronic obstructive pulmonary disease (COPD) were more likely to require bilevel PAP. The investigators postulated that many patients with COPD find exhaling against positive pressure particularly difficult, such that CPAP may worsen hypercapnia and that lowering the expiratory pressure may enhance tolerance. Along these lines, application of bilevel PAP was associated with a lower Paco2 during sleep than during CPAP administration. Although Schafer et al122 observed no association between an obstructive airways disease pattern on spirometry and CPAP failure, those who failed this treatment had significantly higher awake Paco2 and lower Pao2. After 3 months of bilevel PAP there was a significant increase in awake Pao2 and decrease in Paco2.
Although anecdotal evidence suggests that bilevel PAP is better tolerated by some patients and may facilitate therapeutic salvage of patients who are intolerant of CPAP or in whom CPAP is inadequately effective, there are no data to support initial treatment of all OSAH patients with bilevel PAP. A prospective trial in which patients with OSAHS were randomised to receive CPAP or bilevel PAP as initial treatment showed that a greater percentage of the study patients who dropped out or were lost to follow up had been assigned to the CPAP treatment arm than the bilevel PAP treatment arm.123 However, of the patients who completed the study, adherence was comparable in the two groups.
Pressure ramps and autotitrating PAP
One approach to addressing complaints of patients who are unable to fall asleep while wearing CPAP is to use a ramp feature. The ramp, available on most commercially available CPAP devices, allows the clinician to set a rate of rise of delivered pressure such that the patient is provided a window of time on less than therapeutic pressure during which s/he has an opportunity to fall asleep. The patient may reset the pressure to a minimal level and reset the ramp at any time—for example, if s/he gets up in the middle of the night and then wishes to return to sleep. The clinician should be cognisant of the possibility that a patient may constantly reset the pressure ramp throughout the night due to pressure intolerance once the target pressure is achieved. This obviously reflects ineffectiveness of the ramp in alleviating patient intolerance. Such “ramp abuse”124 can be seen by monitoring the pressure profile and adherence records that are generated by many current CPAP devices and which are downloadable for viewing by the clinician. Surprisingly, there have been no published trials evaluating the effectiveness of pressure ramps in improving acceptance and adherence to CPAP.
Autotitrating CPAP (A-CPAP) has recently been introduced in an attempt to minimise the average overnight pressure requirements of patients with OSAHS. These systems are designed to modulate the delivered PAP in response to changes in upper airway resistance which may be associated with varying conditions over the night, including changing sleep stage and body position. Although the specific algorithms are proprietary and differ between manufacturers, these devices are, in general, designed to detect impending upper airway collapse and respond by increasing the delivered pressure.125–135 After a defined period of upper airway stability, the pressure is gradually reduced until instability is once again identified. Thus, unlike fixed pressure CPAP (F-CPAP) which must remain throughout the night at a sufficiently high level to maintain upper airway patency under the most unstable conditions, A-CPAP levels float to accommodate the physiological requirements to maintain upper airway patency. The average overnight pressure required during A-CPAP application is usually the same or lower in autotitrating systems than in conventional F-CPAP.127–129 The putative advantages of A-CPAP include improved patient comfort and adherence to treatment compared with F-CPAP.130–132
The effectiveness of A-CPAP in reducing AHI and the arousal index is similar to F-CPAP,133 as is amelioration of symptoms.129 Data regarding the benefits conferred by A-CPAP regarding acceptance and adherence are mixed, however, with some studies suggesting improvement on A-CPAP and others finding no difference compared with F-CPAP.128,131
Another potentially useful application of A-CPAP is as a tool to enhance the efficiency of pressure titration in the sleep laboratory. In such an application, A-CPAP is employed to determine an F-CPAP prescription for home use. In this regard, a fixed pressure prescription identified from an overnight laboratory trial of A-CPAP (the fixed pressure prescription is defined as that pressure at or below which the patient received for 90% or 95% of the overnight trial period) has been found to approximate pressure prescription derived from manual CPAP titration.133,134 When used in this manner, A-CPAP relieves the sleep technician of the obligation of manually performing the titration. Theoretically, this provides a greater opportunity to address patient comfort and optimise patient education. The use of A-CPAP, applied in an unattended setting to established a long term F-CPAP pressure prescription, has not been adequately studied and its use for that purpose is not currently recommended.135,136
Unresolved problems with A-CPAP include inappropriate pressure adjustments during interface or mouth leaks which impair the algorithms from effectively detecting upper airway instability.134 In addition, A-CPAP has been evaluated only in selected populations with limited or no available data addressing its efficacy or effectiveness in patients with underlying cardiopulmonary disorders, central sleep apnoea, CSB, obesity-hypoventilation, neurological or chest wall abnormalities. Until such data become available, A-CPAP should probably not be used in these patients.135,136 Also, patients with OSAHS who do not snore, as might occur following unsuccessful uvulopalatopharyngoplasty, should not use A-CPAP devices with algorithms that use pressure or airflow vibrations in the algorithm to modulate adjustment of delivered pressure.136
CONCLUSIONS
CPAP is very effective in treating symptoms associated with OSAHS. There are few serious risks and these occur relatively uncommonly. This treatment is generally well tolerated, particularly in view of its intrusive nature. Nonetheless, therapeutic acceptance and adherence remain significant challenges to patients and clinicians. Management of patients who are intolerant of CPAP requires that the clinician identifies the specific source(s) of the intolerance. Problems related to the interface or route of administration demand exploration of mask or nasal prong options. Difficulties related to nasal or pharyngeal discomfort may be addressed by incorporating a non-heated or heated humidifier into the positive pressure circuit or prescribing judicious use of topical nasal steroids. For those patients who do not tolerate CPAP because of discomfort associated with the requisite therapeutic pressure, or those who have waking hypoxaemia or hypercapnia and who may benefit from ventilatory assistance as well as upper airway stabilisation, bilevel PAP may be an acceptable alternative. A-CPAP may also be an option for OSAHS patients who, when awake, are unable to tolerate administration of the therapeutic pressure required during sleep with consequent impairment of the ability to initiate sleep. The advent of PAP for treatment of patients with OSAHS represents an obvious improvement over the treatment of choice which it replaced over two decades ago, tracheostomy. Although PAP remains an imperfect intervention, it has continued to evolve and improve such that patients who would not have been able to use this therapy even in the recent past can benefit from it today.
REFERENCES
Footnotes
-
Supported in part by NIH Training Grant 2-T32-HL07563.
-
M H Sanders is a consultant to Respironics Inc and has a financial interest in BiPAP® and Cflex®.