Article Text
Statistics from Altmetric.com
The unpredictable and potentially lethal course of massive haemoptysis requires prompt resuscitation, airway protection, and correction of coagulopathy. Early investigation with bronchoscopy is recommended for localisation and control of bleeding by the application of topical adrenaline, balloon tamponade, or selective lung intubation. There is increasing acceptance of bronchial artery embolisation as the treatment of choice for acute massive haemoptysis not controlled by conservative treatment, when a bronchial artery can be identified as the source of bleeding. Surgical resection remains the treatment of choice for particular conditions where the bleeding site is localised and the patient is fit for lung resection.
Haemoptysis may be the presenting symptom of a number of diseases,1,2 with an associated mortality ranging from 7% to 30%.3–5 Although fewer than 5% of patients presenting with haemoptysis expectorate large volumes of blood, the explosive clinical presentation and the unpredictable course of life threatening haemoptysis demands prompt evaluation and management. We have reviewed the aetiology of massive haemoptysis and alveolar haemorrhage, with particular reference to current diagnostic and therapeutic strategies.
CASE HISTORY
A 69 year old woman was an emergency admission with large volume haemoptysis which did not settle spontaneously. She had previously undergone a left mastectomy for breast carcinoma. Alveolar shadowing was noted in the left mid zone on the chest radiograph, consistent with recent pulmonary haemorrhage (fig 1A). A thoracic computed thoracic (CT) scan confirmed consolidation and volume loss in the left upper lobe and lingula, but also showed a mass anteriorly eroding through the chest wall, consistent with local recurrence of the breast neoplasm (fig 1B). Pulmonary angiography showed no abnormality, but bronchial angiography identified a trunk that supplied a moderate pathological circulation anteriorly in the left upper lobe in the region of the abnormality on the CT scan. The artery was successfully embolised using polyvinyl alcohol (PVA) foam granules (500–700 μm in diameter, fig 2). The internal mammary artery was also catheterised and a pathological circulation was noted that was occluded using platinum coils (fig 1A) and PVA granules, with no complications and no recurrence of haemoptysis.
(A) Chest radiograph showing previous left mastectomy and left upper lobe and lingular infiltrates due to airway bleeding. A platinum embolisation coil is noted on this post-embolisation radiograph (arrow).(B) CT scan showing a mass lesion (arrow) involving the left anterior chest wall with associated left upper zone consolidation, consistent with recent haemoptysis and local tumour recurrence following the previous mastectomy.
Use of selective bronchial artery embolisation to control massive haemoptysis. (A) Bronchial angiogram showing common trunk and left sided abnormal circulation pre-embolisation, and (B) post-embolisation angiogram showing the left bronchial artery and successful embolisation of abnormal vessels.
DEFINITION
Although there is no generally accepted definition of the volume of blood that constitutes a massive haemoptysis, studies have quoted volumes ranging from 100 ml up to or more than 1000 ml per day.2 As the anatomical dead space of the major airways is 100–200 ml, a more relevant definition of massive haemoptysis is the volume that is life threatening by virtue of airway obstruction or blood loss.5,6
AETIOLOGY
It is important to establish that the lung is the source of bleeding, in part by excluding the nasopharynx or gastrointestinal tract. The most common causes of massive haemoptysis are listed in box 1. Haemoptysis originates from the bronchial and pulmonary circulation in 90% and 5% of cases, respectively.7 Bleeding from the bronchial arteries has the propensity to cause massive haemoptysis as it is a circulation at systemic pressure. Alveolar haemorrhage is a recognised cause of haemoptysis, but rarely causes massive bleeding as the alveoli have the capacity to accommodate a large volume of blood.8 A more common presentation is mild haemoptysis, pulmonary infiltrates, and anaemia.2
Box 1 Causes of massive haemoptysis and alveolar haemorrhage
Infections
-
Mycobacteria, particularly tuberculosis
-
Fungal infections (mycetoma)
-
Lung abscess
-
Necrotising pneumonia (Klebsiella, Staphylococcus, Legionella)
Iatrogenic
-
Swan-Ganz catheterisation
-
Bronchoscopy
-
Transbronchial biopsy
-
Transtracheal aspirate
Parasitic
-
Hydatid cyst
-
Paragonimiasis
Trauma
-
Blunt/penetrating injury
-
Suction ulcers
-
Tracheoarterial fistula
Neoplasm
-
Bronchogenic carcinoma
-
Bronchial adenoma
-
Pulmonary metastases
-
Sarcoma
Haemoptysis in children
-
Bronchial adenoma
-
Foreign body aspiration
-
Vascular anomalies
Vascular
-
Pulmonary infarct, embolism
-
Mitral stenosis
-
Arteriobronchial fistula
-
Arteriovenous malformations
-
Bronchial telangiectasia
-
Left ventricular failure
Coagulopathy
-
Von Willebrand’s disease
-
Haemophilia
-
Anticoagulant therapy
-
Thrombocytopenia
-
Platelet dysfunction
-
Disseminated intravascular coagulation
Vasculitis
-
Behcet’s disease
-
Wegener’s granulomatosis
Pulmonary
-
Bronchiectasis (including cystic fibrosis)
-
Chronic bronchitis
-
Emphysematous bullae
Miscellaneous
-
Lymphangioleiomatosis
-
Catamenial (endometriosis)
-
Pneumoconiosis
-
Broncholith
-
Idiopathic
Spurious
-
Epistaxis
-
Haematemesis
Chronic inflammatory conditions (including bronchiectasis, tuberculosis, lung abscess) and lung malignancies are the most common causes of massive haemoptysis.9,10 Similarly, bleeding may occur from a mycetoma in the presence of cavitating lung disease.11,12 The concurrent development of haemoptysis and menstruation points to a diagnosis of catamenial haemoptysis. The presence of haemoptysis and spontaneous pneumothorax in a woman of childbearing age with diffuse interstitial abnormalities on the chest radiograph should raise the suspicion of lymphangioleiomyomatosis.16
The presence of a saddle nose, rhinitis, or perforated nasal septum may suggest a diagnosis of Wegener’s granulomatosis.17 Features of Behcet’s disease include oral or genital ulceration, uveitis, cutaneous nodules, and pulmonary artery aneurysm which is associated with a 30% 2 year mortality rate.18 Although haematuria may be present in association with Goodpasture’s disease, 5–10% of patients present without clinical evidence of renal disease.8
DIAGNOSTIC PROCEDURES
Sputum should be sent for microbiological investigation, including staining and culture for mycobacteria, and cytological examination if the patient is a smoker and over 40 years of age. Chest radiography may help to identify causative lesions or infiltrates resulting from pulmonary haemorrhage, but fails to localise the lesion in 20–46% of patients with haemoptysis.19 A CT scan may show small bronchial carcinomas or localised bronchiectasis.13,20,21 The use of contrast may help to identify vascular abnormalities such as arteriovenous malformations or aneurysms.14,22 Despite all investigative procedures, the aetiology of haemoptysis is unknown in up to 5–10% of patients.7
MANAGEMENT OF MASSIVE HAEMOPTYSIS
The initial approach to managing life threatening haemorrhage involves resuscitation and protecting the airway (fig 3), the second step is directed at localising the site and cause of bleeding, and the final step involves the application of definitive and specific treatments to prevent recurrent bleeding.
Algorithm for management of massive haemoptysis. *Palliative measures may be appropriate in the setting of advanced malignancy.
Airway protection and resuscitation
All patients with massive haemoptysis should be monitored in an intensive care unit (ICU) or high dependency unit (HDU) and the patient’s fitness for surgery established. Attempts should be made to determine the side of bleeding and the patient positioned with the bleeding side down to prevent aspiration into the unaffected lung. Blood loss should be treated with volume resuscitation, blood transfusion, and correction of coagulopathy. If large volume bleeding continues or the airway is compromised, the patient’s trachea should be intubated with as large an endotracheal tube as is possible to allow adequate suctioning and access for bronchoscopy.2 If the bleeding can only be localised to the right or left lung, unilateral lung intubation may protect the non-bleeding lung.23 For right sided bleeding a bronchoscope may be directed into the left main bronchus which can then be selectively intubated over the bronchoscope with the patient lying in the right lateral position (fig 4). The left lung is then protected from aspiration and selectively ventilated. For a left sided bleeding source the patient is placed in the left lateral position and selective intubation of the right lung may be performed, but this may lead to occlusion of the right upper lobe bronchus.2 An alternative strategy is to pass an endotracheal tube over the bronchoscope into the trachea. A Fogarty catheter (size 14 French/100 cm length) may then be passed through the vocal cords beside the endotracheal tube, directed by the bronchoscope into the left main bronchus and inflated (fig 5). This prevents aspiration of blood from the left lung and the endotracheal tube positioned in the trachea allows ventilation of the unaffected right lung.
Selective intubation of left main bronchus in a case of right sided massive haemoptysis.
Control of left sided massive haemoptysis by tracheal intubation, placement, and inflation of a Fogarty catheter in the left main bronchus.
An alternative strategy for unilateral bleeding is to pass a double lumen endotracheal tube, which allows isolation and ventilation of the normal lung and prevents aspiration from the side involved by bleeding (fig 6).2 However, inserting double lumen tubes should only be performed by experienced operators to avoid the serious consequences of poor positioning.24
Application of a double lumen endotracheal tube for the control of massive haemorrhage. The bronchial lumen is positioned in the left main bronchus to ventilate the left lung and the tracheal lumen is positioned above the carina, allowing ventilation of the right lung while preventing occlusion of the right upper lobe orifice.
Identifying the site and cause of bleeding
Precise localisation of the bleeding site directs definitive treatment. Fibreoptic bronchoscopy and angiography are the modalities of choice to localise the site of bleeding and to allow therapeutic intervention, although the timing of bronchoscopy is controversial.25,26 Early compared with delayed bronchoscopy gives a higher yield for localising the site of bleeding.26 In contrast to mild haemoptysis, localisation of the site of bleeding is essential in the management of massive haemoptysis and urgent bronchoscopy should be considered.7
Fibreoptic bronchoscopy can be performed at the bedside and allows visualisation of more peripheral and upper lobe lesions, but has a limited suction capacity.25,26 Rigid bronchoscopy provides superior suction to maintain airway patency, but it has a limited ability to identify peripheral lesions and does not permit good views of the upper lobes.3 It is usually performed under general anaesthetic but can be performed under local anaesthesia and sedation in experienced hands.27 The techniques can be combined when the fibreoptic bronchoscope is passed through the lumen of the rigid bronchoscope.
Bronchoscopic treatment
Instillation of epinephrine (1:20 000) is advocated to control bleeding, although its efficacy in life threatening haemoptysis is uncertain.2 The topical application of thrombin and thrombin-fibrinogen solutions has also had some success, but further study is required before widespread use can be recommended.28
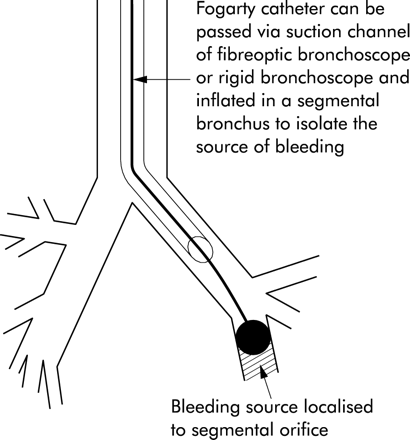
In massive haemoptysis, isolation of a bleeding segment with a balloon catheter may prevent aspiration of blood into the large airways, thereby maintaining airway patency and oxygenation. Having identified the segmental bronchus that is the source of bleeding, the bronchoscope is wedged in the orifice. A size 4–7 Fr 200 cm balloon catheter is passed through the working channel of the bronchoscope and the balloon is inflated in the affected segment, isolating the bleeding site (fig 7).2 A double lumen balloon catheter (6 Fr, 170 cm long) with a detachable valve at the proximal end has recently been designed that passes through the bronchoscope channel and allows the removal of the bronchoscope without any modification of the catheter.29 The second channel of the catheter may also be used to instil vasoactive drugs to help control bleeding. The bronchoscope can then be removed over the catheter, which is left in place for 24 hours. The balloon may be deflated under controlled conditions with bronchoscopic visualisation and the catheter removed if the bleeding has stopped. The prolonged use of balloon tamponade catheters should be avoided to prevent ischaemic mucosal injury and post-obstructive pneumonia. Endobronchial tamponade should only be applied as a temporary measure until a more definitive therapeutic procedure can be deployed.
Placement of a Fogarty catheter guided by fibreoptic bronchoscopy to control massive bleeding from a segmental bronchus.
Neodymium-yttrium-aluminium-garnet (Nd-YAG) laser photocoagulation has been used with some success in the management of massive haemorrhage associated with directly visualised endobronchial lesions.30 However, targeting the culprit vessel with the laser beam can be difficult in the presence of ongoing bleeding.
Bronchial artery embolisation (BAE)
This was first reported by Remy and colleagues in 197331 and is increasingly used in the management of life threatening haemoptysis.20 The procedure involves the initial identification of the bleeding vessel by selective bronchial artery cannulation, and the subsequent injection of particles (polyvinyl alcohol foam, isobutyl-2-cyanoacrylate, Gianturco steel coils or absorbable gelatin pledgets) into the feeding vessel (fig 2). A number of features provide clues to the bronchial artery as the source of bleeding, including the infrequent identification of extravasated dye or the visualisation of tortuous vessels of increased calibre or aneurysmal dilatation.32 The immediate success rates for control of massive haemoptysis is excellent, ranging from 64% to 100%, although recurrent non-massive bleeding has been reported in 16–46% of patients.32–35 Technical failure of BAE occurs in up to 13% of cases and is largely caused by non-bronchial artery collaterals from systemic vessels such as the phrenic, intercostal, mammary, or subclavian arteries.35 Complications of BAE include vessel perforation, intimal tears, chest pain, pyrexia, haemoptysis, systemic embolisation, and neurological complications. When the anterior spinal artery is identified as originating from the bronchial artery, embolisation is often deferred owing to the risk of infarction and paraparesis.32 The development and application of coaxial microcatheter systems allows more selective catheterisation and embolisation of branches of the bronchial arteries, thereby reducing the risk of occluding branches such as the anterior spinal artery.34
Surgical management
Surgery is considered for the management of localised lesions. Surgical mortality ranges from 1% to 50% in different series depending on selection criteria, but bias in the selection of candidates for surgery limits a direct comparison with medical treatment.2 Surgery is contraindicated in patients with inadequate respiratory reserve or those with inoperable lung cancer due to direct thoracic spread. Surgical resection is indicated when BAE is unavailable or the bleeding is unlikely to be controlled by embolisation. It remains the treatment of choice for the management of life threatening haemoptysis due to a leaking aortic aneurysm, selected cases of arteriovenous malformations, hydatid cyst, iatrogenic pulmonary rupture, chest injuries, bronchial adenoma, or haemoptysis related to mycetoma resistant to other treatments.7,23 Pulmonary artery rupture related to the use of pulmonary artery catheters may be temporarily controlled by withdrawing the catheter slightly and reinflating the balloon to compress the bleeding vessel more proximally.36 However, surgical resection of the bleeding vessel is the definitive management.
The onset of massive haemoptysis in a patient with a tracheostomy may be associated with the development of a tracheal-arterial fistula, usually the innominate artery.37 The prompt application of anterior and downward pressure on the tracheal cannula and overinflation of the tracheostomy balloon may help to tamponade the bleeding vessel, and immediate surgical review should be requested. Deflation of the tracheostomy balloon and removal of the tracheal cannula should be performed in a controlled environment.
Other treatment
The oral antifibrinolytic agent tranexamic acid, an inhibitor of plasminogen activation, is frequently used to control recurrent haemoptysis. Intravenous vasopressin has also been used but caution is advised in patients with coexistent coronary artery disease or hypertension. Vasoconstriction of the bronchial artery may also hamper effective BAE by obscuring the site of bleeding, leading to difficulties in cannulation of the artery.2
Systemic antifungal agents have been tried in the management of haemoptysis related to mycetoma, but the results have been poor. By contrast, the direct instillation of antifungal drugs such as amphotericin B with or without N-acetylcysteine or iodine by means of a percutaneous or transbronchial catheter in the cavity has resulted in satisfactory control of haemoptysis in some cases.12,38 This technique should be considered in patients with ongoing bleeding following attempted BAE who are not otherwise fit for surgical resection.
Invasive therapeutic procedures have no role in the management of pulmonary haemorrhage related to coagulopathy, blood dyscrasias, or immunologically mediated alveolar haemorrhage. Appropriate medical treatment is usually sufficient.39 On the rare occasion when an immunologically mediated alveolar haemorrhage leads to massive haemoptysis, the administration of systemic corticosteroids, cytotoxic agents, or plasmapheresis may be useful.8 The long term administration of danazol or gonadotrophin releasing hormone agonists may prove useful in the management of catamenial haemoptysis.40 Radiation therapy has been used in the management of massive haemoptysis associated with vascular tumours or mycetoma by inducing necrosis of feeding blood vessels and vascular thrombosis due to perivascular oedema.41
OUTCOME
Mortality has been closely correlated with the volume of blood expectorated, the rate of bleeding, the amount of blood retained within the lungs and premorbid respiratory reserve, independent of the aetiology of bleeding.1,4 The mortality rate is 58% when the rate of blood loss exceeds 1000 ml/24 hours, compared with 9% if bleeding is less than 1000 ml/hour.7,39 The mortality rate in patients with malignancy is 59%, which increases to 80% in the presence of a combination of malignant aetiology and a bleeding rate of more than 1000 ml/24 hours. A better outcome has been noted for massive haemorrhage due to bronchiectasis, lung abscess, or necrotising pulmonary infections, with a mortality rate of less than 1% in some series.39
Acknowledgments
The authors thank Dr Leslie Mitchell for providing the radiographic and bronchial embolisation images and Dr Stephen Cook and John Sternman for reviewing the manuscript.
The unpredictable and potentially lethal course of massive haemoptysis requires prompt resuscitation, airway protection, and correction of coagulopathy. Early investigation with bronchoscopy is recommended for localisation and control of bleeding by the application of topical adrenaline, balloon tamponade, or selective lung intubation. There is increasing acceptance of bronchial artery embolisation as the treatment of choice for acute massive haemoptysis not controlled by conservative treatment, when a bronchial artery can be identified as the source of bleeding. Surgical resection remains the treatment of choice for particular conditions where the bleeding site is localised and the patient is fit for lung resection.